ImmunoAnalysis. 4:3.
doi: 10.34172/ia.4051
Original Article
Effect of ferric carboxymaltose in the iron deficiency anemia patients undergoing cardiac surgery: A randomized clinical trial
Fateme Ramezani Formal analysis, Investigation, Visualization, Writing – original draft, 1 
Elham Khalaf Adeli Conceptualization, Methodology, Project administration, Supervision, Validation, 2
Karim Shamsasanjan Conceptualization, Methodology, Project administration, Supervision, Validation, 1, * 
Ali Zahed Mehr Methodology, 3
Mostafa Alavi Methodology, 4
Hooman Bakhshandeh Formal analysis, 5
Saeid Hosseini Methodology, 6
Alireza Alizadeh-Ghavidel Methodology, 6
Ali Akbar Pourfathollah Methodology, 7
Author information:
1Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
3Cardiovascular Intervention Research Center, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Sciences, Tehran, Iran
4Cardiac Anesthesia Department, Rajaie Cardiovascular Medical & Research Center, Iran University of Medical Sciences, Tehran, Iran
5Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
6Heart Valve Disease Research Center, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
7Departments of Immunology, Faculty of Medicine, Tarbiat Modares University of Medical Sciences, Tehran, Iran
Abstract
Background:
Iron deficiency is frequent in patients undergoing cardiac surgery. Intravenous iron agents have been used for treating patients with iron deficiency anemia. The present study aimed to investigate the efficacy of ferric carboxymaltose (FCM) among the iron deficiency anemia patient candidates for cardiac surgery.
Methods:
The present non-blinded, randomized, controlled clinical study was performed among two groups of the iron deficiency anemia patients underwent cardiac surgery. The first of whom was infused with a fixed dose of 1000 mg FCM, 3-5 weeks preoperatively, while the second group received no medicine (control). The changes in hemoglobin concentration and biomarkers of iron metabolism were repeated before surgery and 3 to 5 days after surgery. Moreover, the average number of consumed packed cells was assessed.
Results:
In this study, clinical tests, demographic characteristics, and surgery type were similar in two groups. Regarding hemoglobin (Hb) level, a significant difference was demonstrated between FCM-administered group in the preoperative stage (12.1 g/dL, 11.6-12.9) and the control (11.5 g/dL, 10.9-11.8) (P<0.001). Additionally, the preoperative serum ferritin level of FCM group was determined 580 ng/dL (435-787) which significantly differed from that of the control (57 ng/dL, 32-100) (P=0.001).
Conclusion:
The use of FCM is ineffective for preoperative anemia correction in the patients undergoing cardiac surgery and fails to decrease transfusion during surgery in spite of improvement in in iron stores.
Keywords: Iron deficiency anemia, Cardiac surgery, Ferric carboxymaltose, Transfusion
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was supported by the grant No. 80001000 from Tabriz University of Medical Sciences and Barsian Darou company.
Introduction
The consideration of blood transfusion emerges as a crucial therapeutic factor for patients undergoing a range of surgical interventions, with potential implications for their overall well-being. These implications encompass a spectrum of significant complications, such as acute lung and kidney injury, infection, and an extended duration of hospitalization, particularly in the case of individuals undergoing cardiac and non-cardiac surgeries.1-3 However, it is important to acknowledge that blood transfusion remains a common necessity in the field of cardiac surgery, leading to a prevalence of complications that varies considerably, ranging from 15% to 85%.4,5
Preoperative anemia, which is often associated with iron deficiency, is a risk factor for poor outcome in patients undergoing surgery.6 Prevalence of preoperative anemia is notably high among patients undergoing diverse surgical interventions, particularly cardiac surgery, with reported occurrences ranging from 16% to 54%.7-9 The primary causes of anemia in these patients are attributed to iron deficiency, chronic illnesses, and hospital-acquired anemia.10 Importantly, preoperative anemia serves as a fundamental basis for blood transfusion, which has been linked to increased morbidity and mortality rates.11-13
Recent investigations have reported several approaches for managing preoperative anemia. While the efficacy of administering iron for treating preoperative anemia has been established, its effectiveness in the context of cardiac surgery remains unclear.14,15 Despite the availability and ease of oral iron consumption, it is accompanied by undesirable side effects such as nausea, constipation, and abdominal pains.16 Conversely, previous studies have demonstrated that administering intravenous iron at least seven days prior to surgery can augment iron stores and hemoglobin (Hb) concentration.17,18
Ferric carboxymaltose (FCM) is non-dextran containing IV iron with low immunogenic potential, which can be administered in higher doses over a shorter duration of time, and has received favorable feedback from patients. Moreover, it is associated with a low incidence of anaphylactic reactions, as well as fewer adverse effects in comparison to other intravenous iron compounds.19
In this investigation, our ultimate aim is to assess the effectiveness and safety of FCM in rectifying preoperative anemia and reducing for blood transfusion requirements among patients undergoing cardiac surgery, in comparison to individuals in the control group.
Materials and Methods
Study design
This study was a prospective, single-center, non-blinded, randomized, controlled trial performed to evaluate the efficacy and safety of FCM on the iron deficiency anemia patient candidates for cardiac surgery compared to the control group.
Patient selection
All individuals eligible for elective cardiac surgery, specifically coronary artery bypass graft or valve surgery, were thoroughly evaluated in order to determine their suitability for participation in this study. Inclusion criteria comprised patients aged 18 years or older, with Hb levels below 13 g/dL for males and 12 g/dL for females, serum ferritin levels below 100 ng/dL or within the range of 100-299 ng/dL, transferrin saturation below 20%, serum C-reactive protein (CRP) levels below 5 mg/L, and normal serum blood urea nitrogen (BUN) and creatinine (Cr) levels. Additionally, for patients undergoing their first surgery, discontinuation of anti-platelet medications and warfarin was mandated at least five days and 72 hours prior to the surgical procedure, respectively. Conversely, individuals with a known allergy to intravenous iron, liver diseases, coagulation disorders, those requiring re-exploration or cardiac re-operation, as well as pregnant women, were excluded from participation in this study. The CONSORT flow chart illustrating the participant selection process is depicted in Figure 1 for reference.
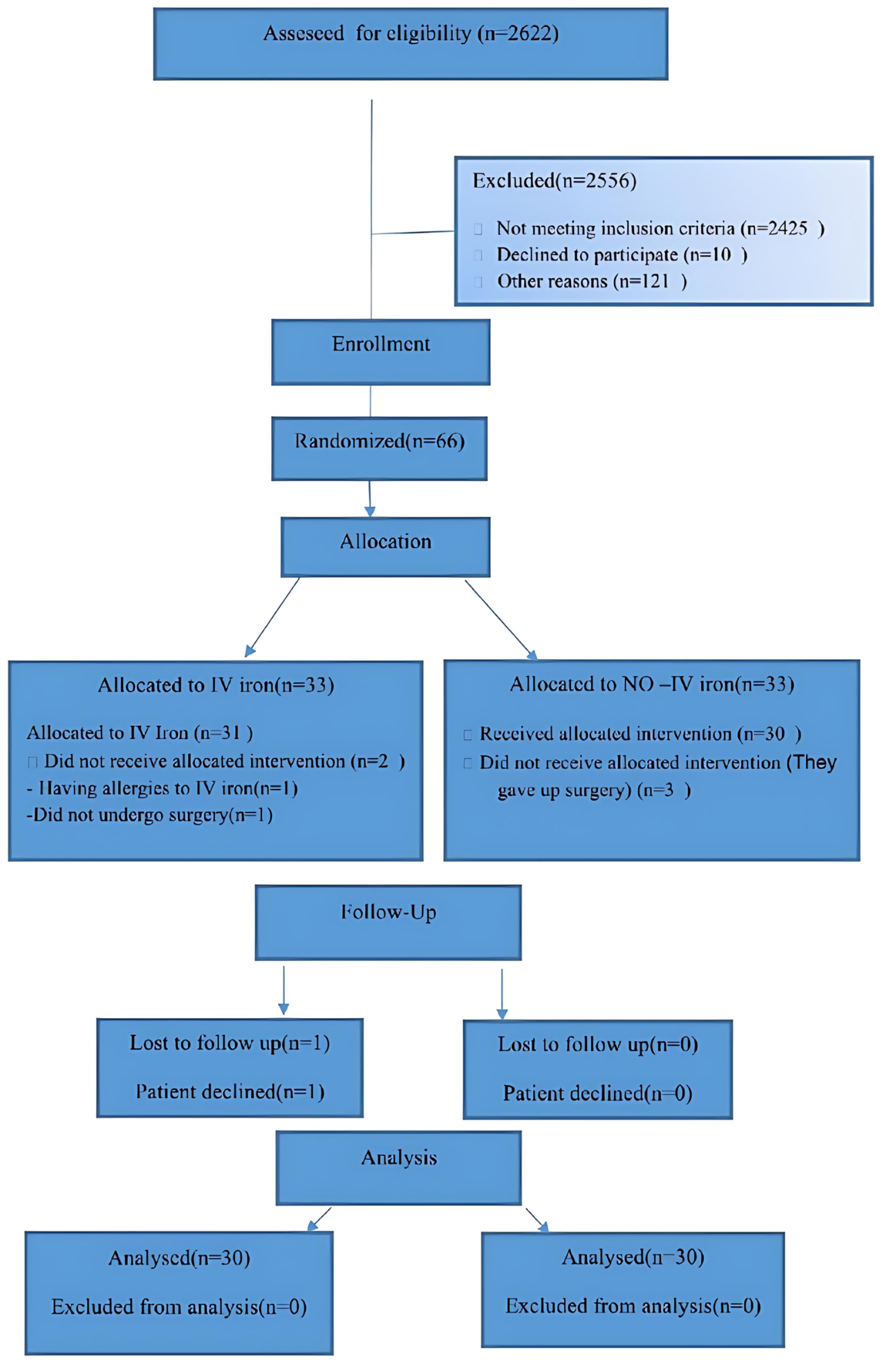
Figure 1.
The CONSORT flowchart representation of randomization and analysis of the trial
.
The CONSORT flowchart representation of randomization and analysis of the trial
Study interventions
The study cohort was allocated into two groups through a randomized process: Group (A) consisted of 30 patients who were administered a standardized preoperative dosage of 1000 mg FCM, while group (B) consisted of 30 patients who did not receive any preoperative medication. Random sampling in both the control and patient groups was conducted using a four-block method.
The laboratory tests such as complete blood count (CBC), serum iron level, serum Ferritin level, BUN, serum Cr level, serum CRP, and serum total iron binding capacity (TIBC) level were performed among anemia subjects. In addition, the patients with confirmed iron deficiency anemia randomized to intravenous iron (FCM) received a fixed dose of 1000 mg FCM. FCM was given by infusing 1000 mg of FCM in 250 mL of normal saline over 15 min, 3 to 5 weeks before cardiac surgery. Subsequently, patients were closely monitored for any potential adverse effects, such as headache, nausea, diarrhea, pruritus, injection site inflammation, hypertension or hypotension, as well as any other untoward reactions, for a duration of 1 hour. The written informed consent was taken from all the patients preoperatively.
Data collection
Three to five weeks after receiving the medicine, the patients were tested for CBC, serum iron and ferritin level, and serum TIBC once before surgery and again 3 to 5 days after surgery. At the same time, randomized iron deficiency anemia patients who received no medicine were considered as the control group. Two groups were assessed and compared regarding packed cell RBC transfusion and LOS at the ICU and the hospital.
Outcomes
The primary outcomes comprised of the following:
-
Amount of Ferritin changes in 2 groups studied before surgery and 3-5 days after surgery, and
-
Amount of Haemoglobin changes in 2 groups studied before surgery and 3-5 days after surgery.
The secondary outcomes comprised of the following:
-
Frequency of patients transfused,
-
Average number of blood units consumed in the operating room, ICU, and total LOS in hospital. and
-
Evaluation of LOS in ICU and hospital
Sample size
There are no complete data regarding the effect of preoperative iron therapy on patients undergoing cardiac surgery. The data analysis was performed using the data based on the increment in ferritin with the use of intravenous iron in patients with colon cancer. The calculations suggested that 30 patients for each group should be sufficient to demonstrate a significant difference. All patients were randomized based on a 1:1 assignment.
Data analysis
The quantitative variables were reported as median (interquartile range), while the qualitative ones were expressed as frequency and percentage. Further, Kolmogorov-Smirnov and non-parametric Mann-Whitney tests were respectively applied for assessing the normality of the test and comparing the medians of quantitative variables in two groups. Furthermore, transfusion was compared between the groups using chi-square test. All P values less than 0.05 were considered as statistically significant. SPSS 25 software was utilized for statistical analyses.
Results
The study included a total of 60 patients divided into two groups, with a mean age of 59.7 ± 1.58 years. Among the participants, 41.7% (n = 25) were male. The clinical characteristics and laboratory test results, including Hb, hematocrit (Hct), serum iron level, serum TIBC, transferrin saturation, and serum ferritin level, were determined and compared between the two groups at the beginning of the study; however, no significant differences were observed in these parameters. Moreover, the distribution of surgery types did not show any significant differences between the two groups (Table 1).
Table 1.
Clinical and demographic characteristics of the patients under study
|
|
No-IV iron
|
FCM
|
P
value
|
| Gender |
|
|
0.2 |
| Male |
15 (50%) |
10 (33.3%) |
|
| Female |
15 (50%) |
20 (66.7%) |
|
| Age (y) |
66 (61.5-70) |
65(61 -68.5) |
0.4 |
| Surgical procedure |
|
|
0.11 |
| Coronary |
16 (53.3%) |
10 (33.3%) |
|
| Valvular |
9 (30%) |
17 (56.7%) |
|
| (Coronary + Valvular) |
5 (16.7%) |
3 (10%) |
|
| Hb (g%) |
11.5 (10.9-11.8) |
11.5 (10.5-11.8) |
0.64 |
| Hct (%) |
35.4 (34.8-37.02) |
35.2 (31.8-36.4) |
0.2 |
| MCV (fL) |
81.1 (79.5-82.8) |
80.1 (75.9-83.9) |
0.2 |
| MCH (pg) |
27.5 (26.1-28.2) |
26.8 (24.9-27.5) |
0.07 |
| MCHC (%) |
32 (30.6-32.8) |
32.4 (31.2-33.1) |
0.3 |
| RDW (%) |
13.2 (12.2-15.1) |
13.5 (12.9-15.4) |
0.1 |
| RBC count |
4.3 (4.2-4.5) |
4.5 (4.1-4.7 ) |
0.3 |
| WBC count |
6.8 (6.5-7.5) |
6.7 (5.3-7.5) |
0.6 |
| Serum iron level (ng/dL) |
38 (25-47) |
39 (26-49) |
0.1 |
| TIBC (mg/dL) |
322 (312-388.7) |
343 (298.5-378.5) |
0.64 |
| Ferritin (ng/dL) |
57 (32-100) |
66 (19.1-113) |
0.82 |
FCM: ferric carboxymaltose, TIBC: Total iron-binding capacity.
The data are expressed as percentage, absolute number, and median or interquartile range.
Table 2 presents a comprehensive comparison of laboratory test results obtained from subjects belonging to the FCM group and the control group at both preoperative and postoperative stages. The data reveals noteworthy disparities between the two groups in terms of preoperative levels of Hb, Hct, serum iron, and serum ferritin. Specifically, the FCM group exhibited significantly higher values in these parameters compared to the control group, with P values less than 0.05. Moving on to the postoperative stage, subjects who received FCM demonstrated elevated levels of iron and ferritin when compared to the control group, and this difference was statistically significant (P < 0.05) (Figure 2). However, no substantial variation was observed between the two groups in terms of Hb and Hct levels during this stage (Figure 3).
Table 2.
Comparing the median (interquartile range) of Hb, Hct, iron parameters, TIBC, and ferritin after receiving medicine in the pre- and postoperative stages
|
|
|
Hb (g/dL)
|
Hct (%)
|
MCV (fL)
|
MCH (pg)
|
MCHC (%)
|
RDW (%)
|
RBC count (x10*6/µL)
|
WBC count (x10*3/µL)
|
| Preoperative |
No-IV iron |
11.5
(10.9-11.8) |
35.4
(33.7-37) |
81.1
(79.5-82.8) |
27.5
(26.1-28.2) |
32
(30.6-32.8) |
13.2
(12.2-15.1) |
4.3
(4.2-4.5) |
6.8
(6.5-7.5) |
| FCM |
12.1
(11.6-12.9) |
37.7
(35.6-40.2) |
80.9
(74.1-85.9 |
26.2
(24.3-28.9) |
32.6
(31.4-33.3) |
13.5
(12.9-15) |
4.6
(4.3-4.9) |
6.5
(5.7-7.1) |
|
P value |
0.001 |
0.002 |
0.04 |
0.2 |
0.2 |
0.003 |
0.004 |
0.3 |
| Postoperative |
No-IV iron |
10
(8.8-10.5) |
30.4
(28.2-34.1) |
82.8
(81.8-86.7) |
27
(26.4-27.7) |
31.9 (31.4-32.5) |
12.3 (12-13.2) |
3.6
(3.5-3.8) |
7.4
(6.6-8.1) |
| FCM |
9.6
(9-10.5) |
30.1
(28.2-33.6) |
83.4
(79.4-87.6) |
27
(25.2-28.5) |
32.1 (30.9-32.5) |
13.5
(12.9-14.7) |
3.5
(3.2-3.9) |
7.9
(6.8-10.1) |
|
P value |
0.8 |
0.8 |
0.8 |
0.7 |
0.4 |
0.001 |
0.4 |
0.06 |
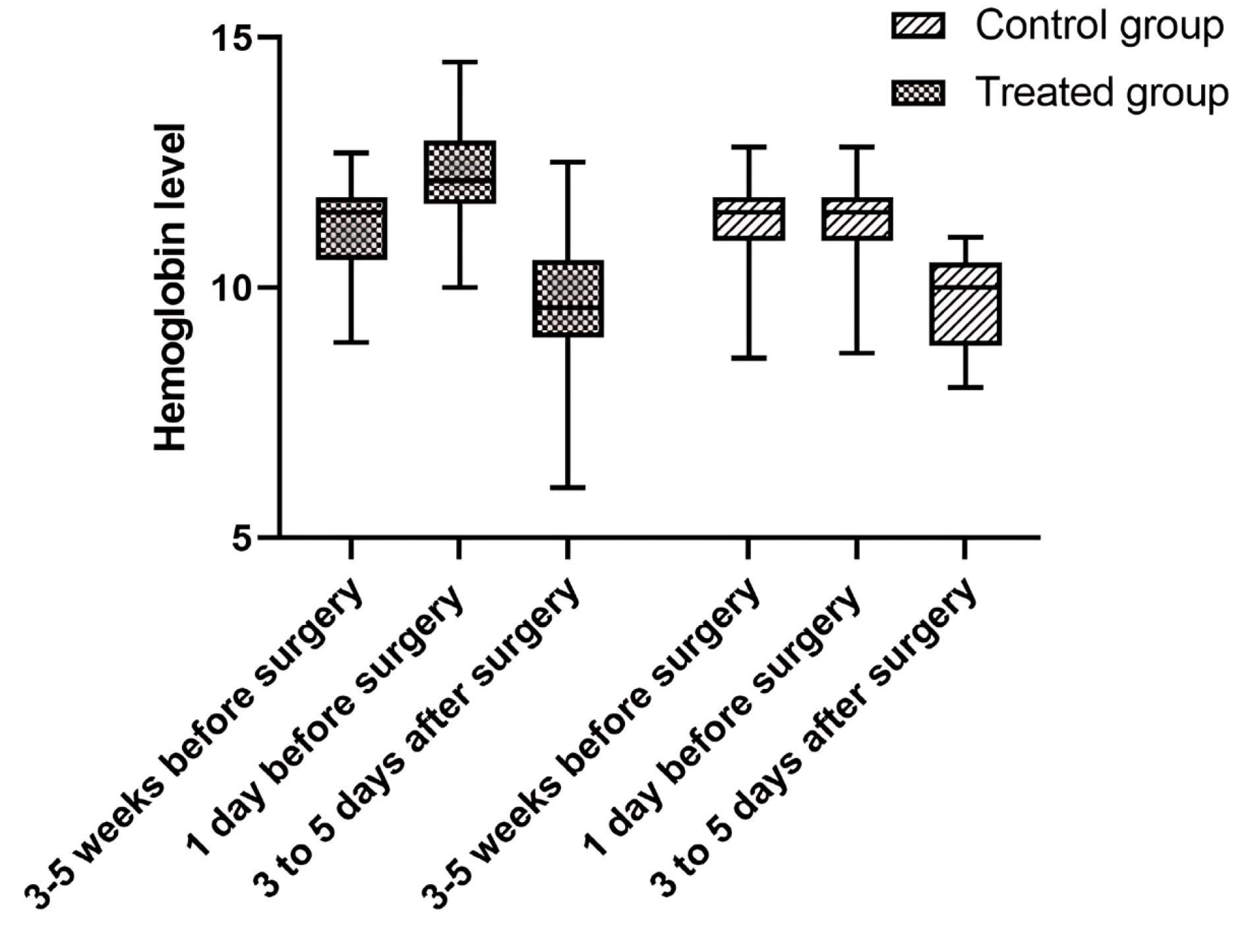
Figure 2.
Ferritin levels 3-5 week before treatment, 1 day before treatment and 3-5 days after-surgery in the Ferric carboxymaltose and control groups
.
Ferritin levels 3-5 week before treatment, 1 day before treatment and 3-5 days after-surgery in the Ferric carboxymaltose and control groups
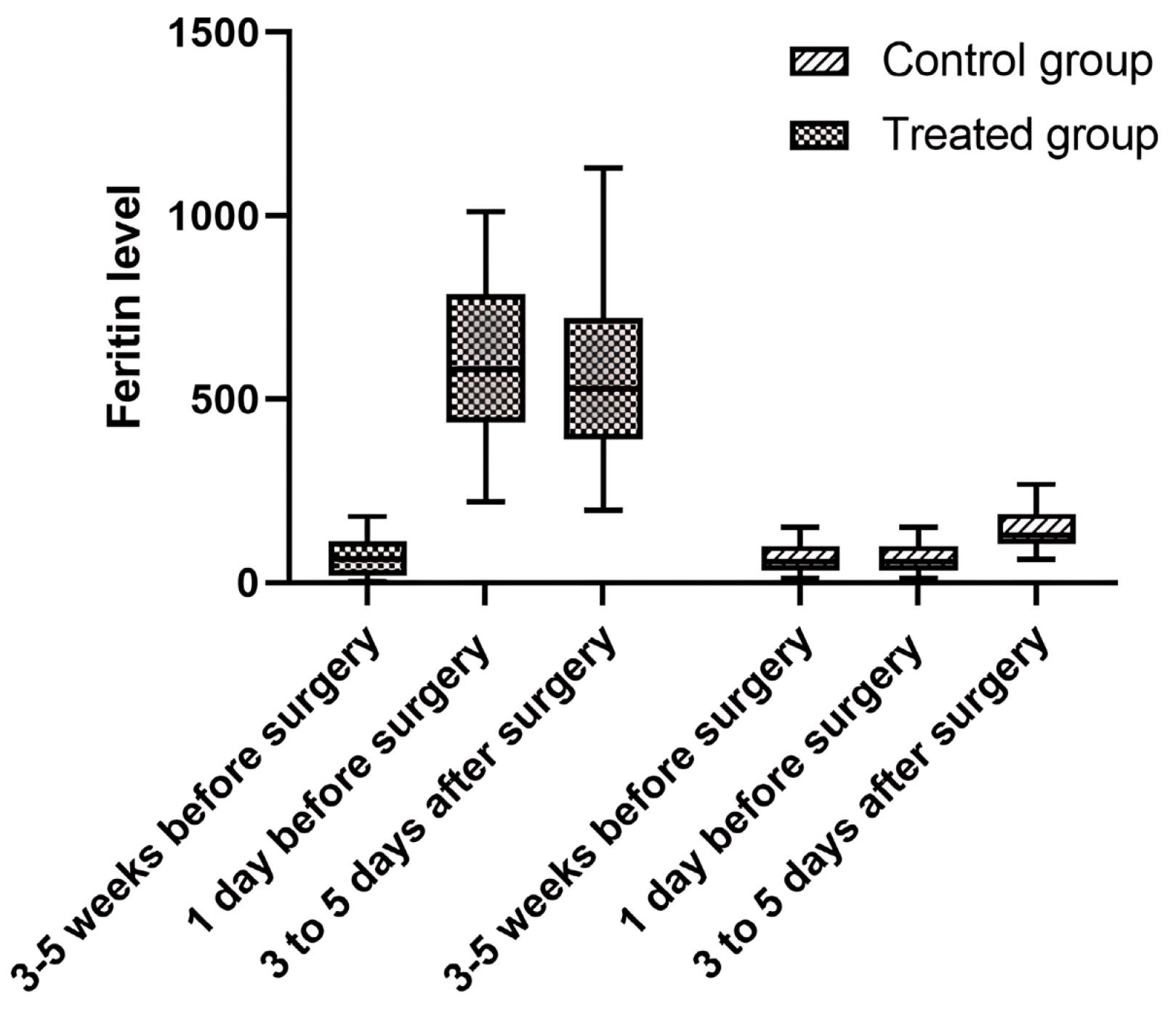
Figure 3.
Hemoglobin levels 3-5 week before treatment, 1 day before treatment and 3-5 days after-surgery in the Ferric carboxymaltose and control groups
.
Hemoglobin levels 3-5 week before treatment, 1 day before treatment and 3-5 days after-surgery in the Ferric carboxymaltose and control groups
To assess the impact of the medication on blood transfusion, the necessity for red blood cell (RBC) products was evaluated during and after surgery in both groups. The analysis indicated that the administration of FCM did not significantly affect the need for blood transfusion among patients (P value > 0.05). However, it is worth noting that the FCM group exhibited a lower demand for blood transfusion in the intensive care unit (ICU) compared to the control group, and this difference was statistically significant (P value < 0.05) (Table 3).
Table 3.
Comparing the RBC product (packed cell) transfusion in the operating room, ICU, and total LOS in hospital
|
|
No-IV iron
|
FCM
|
P
value
|
| Need for RBC transfusion overall (%) |
100% (30) |
90% (27) |
0.2 |
| Need for RBC transfusion in OR (%) |
100% (30) |
90% (27) |
0.2 |
| Need for RBC transfusion in ICU (%) |
80% (24) |
43.3% (13) |
< 0.05 |
| Median units of RBC transfused (overall) |
3 (3-5) |
1(1-2) |
0.4 |
| Median units of RBC transfused in OR |
2 (1.75-3) |
1(1-1) |
0.2 |
| Median units of RBC transfused in ICU |
1.5(1-2) |
0 (0-1) |
< 0.05 |
OR: operating room, FCM: ferric carboxymaltose, RBC: red blood cell, ICU: intensive care unit.
The data are reported as percentage and median or interquartile range.
Additionally, there were no significant differences observed between the two groups in terms of the length of stay (LOS) in the ICU (P > 0.05). However, the FCM group experienced a shorter duration of hospitalization compared to the control group (P < 0.05) (Table 4).
Table 4.
Comparing LOS in ICU and hospital between two groups
|
|
No-IV iron
|
FCM
|
P
value
|
| Stay in ICU (days) |
3 (2-3.2) |
3 (3-3) |
0.9 |
| Stay at hospital (days) |
10.5 (8.7-13.2) |
9 (7.7-10.2) |
0.01 |
FCM: ferric carboxymaltose.
The data are presented as median or interquartile range.
Out of the 30 patients who received intravenous FCM, only one individual experienced side effects in the form of hives and itching on the arm following the infusion of the medication. This particular patient was subsequently excluded from the study based on the predetermined exclusion criteria.
Discussion
Our study findings have unveiled evidence regarding the salutary effects of preoperative iron therapy on patients undergoing cardiac surgeries. Notably, a significant augmentation in Hb levels, serum ferritin concentrations, and serum iron levels was observed following the administration of iron therapy. Moreover, a noteworthy reduction in serum TIBC was discerned, underscoring the favorable impact of iron supplementation on iron metabolism.
Intriguingly, our investigation revealed that intravenous FCM injection did not engender a discernible decrease in the need for blood transfusion among patients afflicted with iron deficiency anemia prior to cardiac surgery. However, a profound disparity between the treatment and control groups manifested concerning secondary outcomes, notably encompassing the duration of hospitalization and the extent of transfusion requirements within the ICU.
These compelling findings align harmoniously with precedent clinical trials conducted by esteemed researchers such as Padmanabhan et al,20 Garrido-Martín et al21 and Urena et al,22 who diligently explored the intricate interplay of iron injection in rectifying anemia both pre- and post-surgical interventions in candidates for cardiac surgery. Remarkably, these studies consistently corroborate our findings, elucidating that iron supplementation precipitates a substantial elevation in serum ferritin levels, thereby substantiating the efficacy of iron therapy in optimizing iron stores. In addition, we indicated a significant difference in the level of serum Hb after receiving the drug before surgery in the FCM group compared to the control group. These findings are based on the study of a prospective randomized trial conducted by Spahn23 and a retrospective cohort study by Peel et al.24 In both studies, intravenous iron increased the level of serum Hb and decreased transfusion.
In our study, despite the significant difference in the Hb level in the group receiving intravenous iron compared to the control group, we did not see a reduction in transfusion during surgery, although there was a significant difference in the amount of transfusion in the ICU in the group receiving intravenous iron compared to the control group. This may be because there are different surgeons in the operating room and they do not operate according to the blood transfusion guidelines. The start of injection of RBC product in patients undergoing heart surgery is Hb of 8g/dL or less, but some surgeons do not operate according to the guidelines. Therefore, it cannot be said precisely that the FCM is not effective on transfusion.25
The efficacy of iron therapy in the specific context of cardiac surgeries remains a subject of ongoing investigation despite its well-documented beneficial effects in various surgical settings. Our study delves into this intricate domain, shedding light on important aspects of its effectiveness. Our study discerns a notable trend among patients with absolute iron deficiency anemia, indicating a favorable hematopoietic response to iron therapy. This is evident from the substantial increase in Hb levels observed following the administration of intravenous iron within the perioperative window of 3 to 5 weeks. Such findings highlight the potential of iron therapy to address the underlying iron deficiency contributing to preoperative anemia in this specific patient population. In contrast, patients afflicted with functional iron deficiency, characterized by elevated hepcidin levels, unfortunately did not exhibit the same hematopoietic benefits from iron therapy. This observation underscores the multifactorial nature of preoperative anemia and emphasizes the limitations of iron therapy as a standalone intervention for addressing functional iron deficiency anemia. It is important to note that functional iron deficiency is a common underlying etiology of preoperative anemia.26,27
These findings underscore the need for a comprehensive approach that takes into account the multifaceted etiology of preoperative anemia, including functional iron deficiency. Further research is necessary to explore additional interventions or therapeutic strategies that can effectively address the complex mechanisms underlying functional iron deficiency anemia in the context of cardiac surgeries.
Conclusion
Our study findings indicate that there was no significant increase in Hb levels at the conclusion of the study. However, a notable difference of 0.6 gr/dL was observed between the treatment group receiving iron therapy and the control group. These results suggest that patients affected by absolute iron deficiency anemia may exhibit a more favorable response to iron therapy, potentially leading to a reduction in transfusion requirements compared to the control group. Further research is warranted to explore the underlying mechanisms and optimize the use of iron therapy in this specific patient population.
Acknowledgements
We would like to be grateful to Tabriz University of Medical Sciences and Barsian Darou company for supporting this study by grant No. 80001000.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
The present study was conducted in Shahid Rajaei Cardiovascular, Medical, and Research Center during the one-year period with the ethical No. IR.TBZMED.REC.1399.486 (October 2018 to September 2019).
References
- Muñoz M, Gómez-Ramírez S, Campos A, Ruiz J, Liumbruno GM. Pre-operative anaemia: prevalence, consequences and approaches to management. Blood Transfus 2015; 13(3):370-9. doi: 10.2450/2015.0014-15 [Crossref] [ Google Scholar]
- Nalla BP, Freedman J, Hare GM, Mazer CD. Update on blood conservation for cardiac surgery. J Cardiothorac Vasc Anesth 2012; 26(1):117-33. doi: 10.1053/j.jvca.2011.07.024 [Crossref] [ Google Scholar]
- Gani F, Cerullo M, Ejaz A, Gupta PB, Demario VM, Johnston FM. Implementation of a blood management program at a tertiary care hospital: effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg 2019; 269(6):1073-9. doi: 10.1097/sla.0000000000002585 [Crossref] [ Google Scholar]
- Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, Herruzo-Avilés A, Camacho-Laraña P, Garnacho-Montero J. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001; 119(5):1461-8. doi: 10.1378/chest.119.5.1461 [Crossref] [ Google Scholar]
- Reddy SM, Talwar S, Velayoudam D, Gharde P, Mallick V, Jha RK. Multi-modality blood conservation strategy in open-heart surgery: an audit. Interact Cardiovasc Thorac Surg 2009; 9(3):480-2. doi: 10.1510/icvts.2009.203034 [Crossref] [ Google Scholar]
- Banerjee S, McCormack S. Intravenous Iron Preparations for Patients Undergoing Elective Surgery: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2019.
- Hung M, Besser M, Sharples LD, Nair SK, Klein AA. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients. Anaesthesia 2011; 66(9):812-8. doi: 10.1111/j.1365-2044.2011.06819.x [Crossref] [ Google Scholar]
- Padmanabhan H, Aktuerk D, Brookes MJ, Nevill AM, Ng A, Cotton J. Anemia in cardiac surgery: next target for mortality and morbidity improvement?. Asian Cardiovasc Thorac Ann 2016; 24(1):12-7. doi: 10.1177/0218492315618032 [Crossref] [ Google Scholar]
- Hogan M, Klein AA, Richards T. The impact of anaemia and intravenous iron replacement therapy on outcomes in cardiac surgery. Eur J Cardiothorac Surg 2015; 47(2):218-26. doi: 10.1093/ejcts/ezu200 [Crossref] [ Google Scholar]
- Tankard KA, Park B, Brovman EY, Bader AM, Urman RD. The impact of preoperative intravenous iron therapy on perioperative outcomes in cardiac surgery: a systematic review. J Hematol 2020; 9(4):97-108. doi: 10.14740/jh696 [Crossref] [ Google Scholar]
- Hallward G, Balani N, McCorkell S, Roxburgh J, Cornelius V. The relationship between preoperative hemoglobin concentration, use of hospital resources, and outcomes in cardiac surgery. J Cardiothorac Vasc Anesth 2016; 30(4):901-8. doi: 10.1053/j.jvca.2016.02.004 [Crossref] [ Google Scholar]
- Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med 2012; 185(10):1049-57. doi: 10.1164/rccm.201110-1915CI [Crossref] [ Google Scholar]
- Gupta PK, Sundaram A, Mactaggart JN, Johanning JM, Gupta H, Fang X. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg 2013; 258(6):1096-102. doi: 10.1097/SLA.0b013e318288e957 [Crossref] [ Google Scholar]
- Ralley FE. Erythropoietin and intravenous iron in PBM. Transfus Apher Sci 2014; 50(1):16-9. doi: 10.1016/j.transci.2013.12.007 [Crossref] [ Google Scholar]
- Petis SM, Lanting BA, Vasarhelyi EM, Naudie DD, Ralley FE, Howard JL. Is there a role for preoperative iron supplementation in patients preparing for a total hip or total knee arthroplasty?. J Arthroplasty 2017; 32(9):2688-93. doi: 10.1016/j.arth.2017.04.029 [Crossref] [ Google Scholar]
- Covic A, Mircescu G. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study. Nephrol Dial Transplant 2010; 25(8):2722-30. doi: 10.1093/ndt/gfq069 [Crossref] [ Google Scholar]
- Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth 2011; 107(3):477-8. doi: 10.1093/bja/aer242 [Crossref] [ Google Scholar]
- Cladellas M, Farré N, Comín-Colet J, Gómez M, Meroño O, Bosch MA. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol 2012; 110(7):1021-6. doi: 10.1016/j.amjcard.2012.05.036 [Crossref] [ Google Scholar]
- Klaire E, Nancy T, Andrea A, Atif K, Shahed A. Efficacy and safety profile of single dose IV FCM in the management of renal anaemia-a single centre experience. Nephrol Dial Transplant 2013; 28(1):363-4. [ Google Scholar]
- Padmanabhan H, Siau K, Nevill AM, Morgan I, Cotton J, Ng A. Intravenous iron does not effectively correct preoperative anaemia in cardiac surgery: a pilot randomized controlled trial. Interact Cardiovasc Thorac Surg 2019; 28(3):447-54. doi: 10.1093/icvts/ivy226 [Crossref] [ Google Scholar]
- Garrido-Martín P, Nassar-Mansur MI, de la Llana-Ducrós R, Virgos-Aller TM, Rodríguez Fortunez PM, Ávalos-Pinto R. The effect of intravenous and oral iron administration on perioperative anaemia and transfusion requirements in patients undergoing elective cardiac surgery: a randomized clinical trial. Interact Cardiovasc Thorac Surg 2012; 15(6):1013-8. doi: 10.1093/icvts/ivs344 [Crossref] [ Google Scholar]
- Urena M, Del Trigo M, Altisent OA, Campelo-Prada F, Regueiro A, DeLarochellière R. Combined erythropoietin and iron therapy for anaemic patients undergoing transcatheter aortic valve implantation: the EPICURE randomised clinical trial. EuroIntervention 2017; 13(1):44-52. doi: 10.4244/eij-d-16-00591 [Crossref] [ Google Scholar]
- Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019; 393(10187):2201-12. doi: 10.1016/s0140-6736(18)32555-8 [Crossref] [ Google Scholar]
- Peel JK, Trudeau J, Tano R, Jadunandan S, Callum J, Moussa F. Determining optimal treatment to correct preoperative anemia and reduce perioperative allogeneic blood transfusions in cardiac surgery: a retrospective cohort study. J Cardiothorac Vasc Anesth 2021; 35(9):2631-9. doi: 10.1053/j.jvca.2020.12.044 [Crossref] [ Google Scholar]
- Klein A, Agarwal S, Cholley B, Fassl J, Griffin M, Kaakinen T. A survey of patient blood management for patients undergoing cardiac surgery in nine European countries. J Clin Anesth 2021; 72:110311. doi: 10.1016/j.jclinane.2021.110311 [Crossref] [ Google Scholar]
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352(10):1011-23. doi: 10.1056/NEJMra041809 [Crossref] [ Google Scholar]
- Abraham J, Sinha R, Robinson K, Scotland V, Cardone D. Aetiology of preoperative anaemia in patients undergoing elective cardiac surgery-the challenge of pillar one of Patient Blood Management. Anaesth Intensive Care 2017; 45(1):46-51. doi: 10.1177/0310057x1704500107 [Crossref] [ Google Scholar]