ImmunoAnalysis. 4:2.
doi: 10.34172/ia.4055
Mini Review
Novel Approaches Used for Decreasing Apoptosis Rate in Ovarian Tissue Cryopreservation and Transplantation
Mahsa Rezaei Zarnaghi Investigation, Writing – original draft, Writing – review & editing, 
Zahra Bahroudi Software, Writing – original draft,
Melika Izadpanah Investigation, Validation, Writing – original draft, Writing – review & editing, 
Abbas Majdi Seghinsara Visualization,
Ali Abedelahi Conceptualization, Methodology, Supervision, * 
Author information:
Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Apoptosis is the main cause of atresia in ovarian follicles. In oxidative stress (OS) conditions, an imbalance between pro-apoptotic and anti-apoptotic genes can increase the apoptosis rate. In some diseases, such as cancers, patients lose fertility due to chemotherapy and radiotherapy. One way to maintain fertility in these patients is ovarian tissue cryopreservation and transplantation (OTC/T). Studies show that OTC/T also increases apoptosis due to hypoxia and ischemia but fortunately new findings show that applying new approaches could decrease apoptosis in these procedures considerably. This article reviewed follicular atresia, apoptosis during cryopreservation and transplantation, and factors affecting the reduction of apoptosis.
Keywords: Ovarian transplantation, Apoptosis, Ovarian cryopreservation, Antioxidant, Cell therapy
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was supported by Tabriz University of Medical Sciences.
Introduction
The ovaries are essential organs for maintaining fertility in women whose follicles are constantly growing and degenerating.1 Anticancer therapies often cause ovarian failure and infertility because the ovarian follicles storage is susceptible to the effects of radiotherapy and chemotherapy.2,3 Chemotherapy causes damage to the vascular network and stromal cells of the ovary. It reduces the reserve of the primary follicle by disrupting the signaling pathways involved in quiescence maintenance and increasing apoptosis.4 Infertility caused by cancer treatment causes anxiety and distress in many cancer survivors. There are many ways to maintain fertility, including oophoropexy or ovarian transposition, embryos cryopreservation, oocyte cryopreservation and ovarian tissue cryopreservation and transplantation (OTC/T).5 Oophoropexy or ovarian transposition is a surgical manner, which mostly done by laparoscopic procedure, used for ovarian tissue preservation and fertility in patients treated with radiotherapy.3,6-9 Embryo cryopreservation needs the gonadotropins stimulation, which enhanced in estradiol levels that have opposite effect in therapy of estrogen sensitive tumors.10 However, This method is not suitable for patients who need to start chemotherapy immediately, troublous as for achieving oocytes, which need to partner or donor sperm and is improper for patients before puberty.11,12 Oocyte cryopreservation is more vulnerable to the risk of damaging intracellular ice formation because of wide surface area to volume ratio and low water penetrance.13,14 OTC/T as a new technology to maintain fertility is developing rapidly. Vitrification, slow freezing, and ultra-fast freezing are standard methods for ovarian tissue cryopreservation (OTC).15,16 The successful cryopreservation and transplantation of ovarian tissue is obtained by some following steps; first the ovarian tissue is exposed to cryoprotectants (CPAs) such as ethylene glycol and dimethyl sulfoxide. In the following, freezing and cooling should be done by the liquid nitrogen (-196 °C). The next step is tissue thawing and rewarming for CPA removal by thawing solutions, final step is transplanting ovarian tissue to resume its activity. OTC/T is a faster method than oocyte and embryo cryopreservation and does not require lengthy processes such as ovarian stimulation and finding donated sperm. In addition to these benefits, OTC/T can restore follicular storage and continuing the secretion of ovarian endocrine hormones by maintaining ovarian tissue.17-21 Some agents can decrease follicle surviving in OTC/T. Ischemia is the most critical injury during ovarian tissue transplantation ultimate to depletion of cellular energy storages, induction of apoptosis, accumulation of toxic metabolites and hypoxia in the ovarian tissue.22 OTC/T without vascular anastomosis causes hypoxia and ischemia lasting up to 48 hours in rodents23 and five days in humans24 and thereby leads to loss of 50% to 65% of primordial follicles.4,25,26 Moreover, osmotic shock and oxidative stress (OS) increased morphological changes and mitochondrial damages to the follicles, which ultimately cause apoptosis and cell death during OTC/T.27,28 Recent studies have suggested that apoptosis is a main cause of failure in OTC/T.
Apoptosis is the underlying mechanism of ovarian tissue changes such as plasma membrane blebbing, cell shrinkage, nuclear condensation and fragmentation and also DNA fragmentation.29 In general, apoptosis has two intrinsic and extrinsic pathways (Figure 1). The intrinsic pathway or mitochondrial pathway can be initiated due to the presence or absence of certain factors. For example, the lack of hormones and growth factors or exposure of cells to hypoxia, radiation, and reactive oxygen species (ROS) can cause apoptosis. The intrinsic pathway in response to stressful conditions is activated by the release of cytochrome-c from the mitochondria and the formation of apoptosome as well as caspase-9 activation.30 Extracellular signals induce external apoptosis. The binding of the ligand to specific receptors leads to the construction of the death initiation-signaling complex and activates caspase-8. Eventually, cell death occurs with the activation of downstream caspases such as caspase-3.31,32
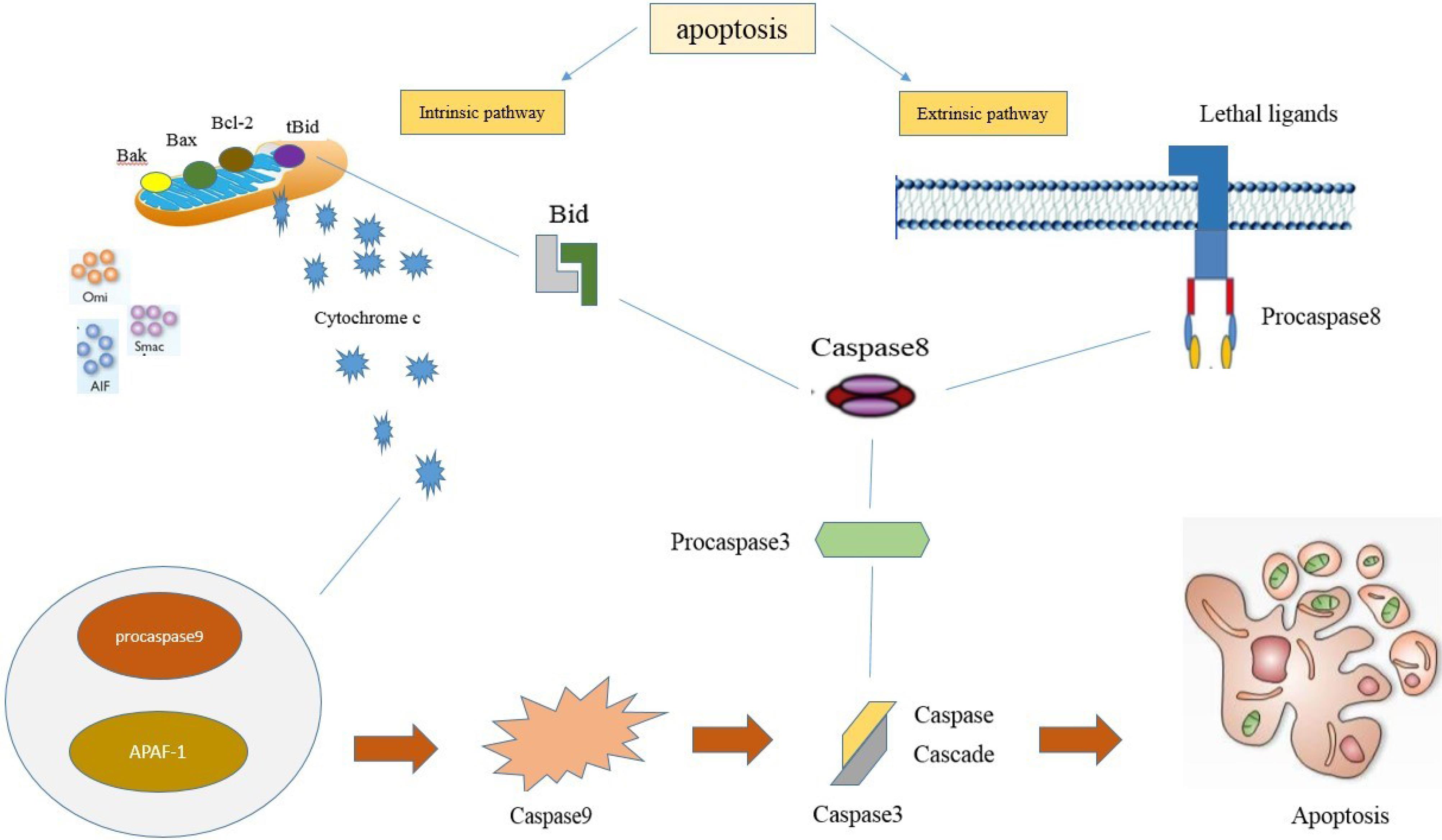
Figure 1.
The component of apoptosis primarily comprises of two center pathways induces apoptosis; extrinsic pathway and intrinsic pathway. extrinsic pathway alludes to extracellular ligands are attached to the extracellular domain of the transmembrane receptors and the intrinsic pathway may be a mitochondrial-mediated pathway. Both of these apoptotic pathways can be led to same terminal (execution pathway)
.
The component of apoptosis primarily comprises of two center pathways induces apoptosis; extrinsic pathway and intrinsic pathway. extrinsic pathway alludes to extracellular ligands are attached to the extracellular domain of the transmembrane receptors and the intrinsic pathway may be a mitochondrial-mediated pathway. Both of these apoptotic pathways can be led to same terminal (execution pathway)
Apoptosis in physiological condition of ovarian tissue
The ovary is a dynamic organ that quickly removes excess and faulty germ cells to assure the ovulation of viable and high-quality ova for fertilization. During fetal development, millions of germ cells are present in the ovarian, but the ovary loses most of them before they start working. Apoptosis may play essential roles in mammalian ovarian germ cell depletion either alone or with autophagy.33,34 Ovarian reserve is dependent to genetics, age, and environment. A baby girl is born with about 2 million primordial follicles in the ovary, but by the time menarche occurs, about 400 000 follicles remain due to natural follicular atresia. In her mid-30s, due to the increased oocyte depletion, the number of follicles is decreased to around 25 000.35 Follicular apoptosis was first reported by Flemming in 1885 based on morphological changes36; and occurs in ovarian oocytes, granulosa, and luteal cells and is mainly restricted to the granulosa cells (GCs) of developing follicles in adult ovaries.37 Apoptosis occurs as a physiological process at all stages of follicular development, especially in luteal regression and follicular atresia. Most follicles undergo apoptosis in the antral stage.38 Generally, apoptosis and degeneration of GCs lead to follicular atresia (Figure 2). One study showed that apoptosis in GCs depends on an imbalance between progesterone and estradiol in the follicular fluid. The level of Insulin growth factor-I can play a role in controlling apoptosis in GCs during follicular atresia.39 Several molecular pathways appear to be involved during ovarian apoptosis, including the Bcl-2 family, caspases, Tumor necrosis factor, and transforming growth factor beta (TGF-β) proteins.40 Studies on miRNAs in the ovary have also shown that miRNAs are involved in the process of follicular atresia and GC apoptosis. The prominent miRNA families and clusters, including the let-7 family, miR-183-96-182, miR-17-92, and miR-23-27-24 clusters are involved in follicular atresia.41 MiR-146b as a pro-apoptotic factor via suppressing cytochrome P450 family 19 subfamily A member 1 (CYP19A1) expression promotes ovarian GC apoptosis.42 A study showed that miR-1275 via impairing the liver receptor homolog (LRH)-1/CYP19A1 axis and inhibiting estradiol (E2) release could promote GC apoptosis and initiate follicular atresia in ovaries.43
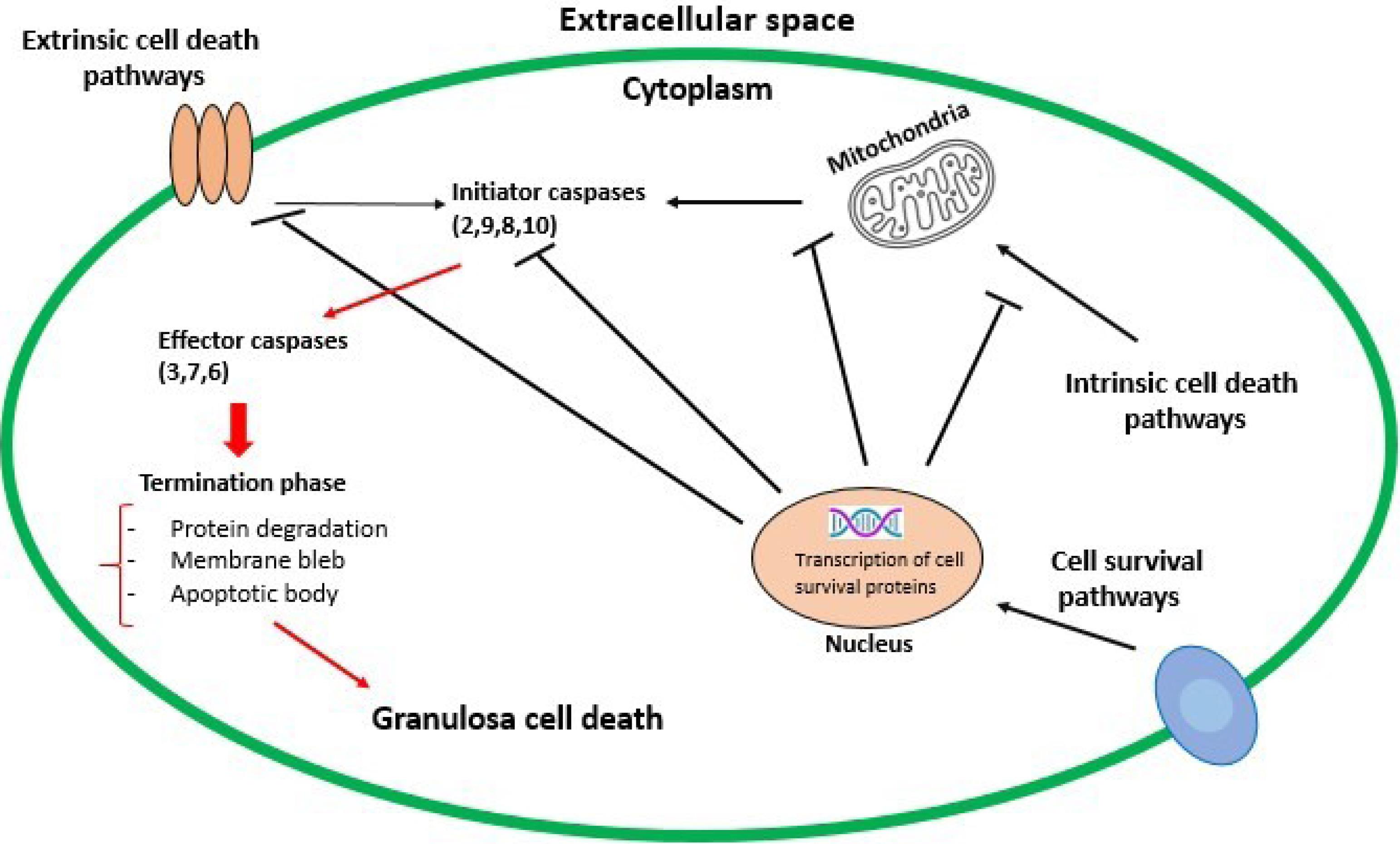
Figure 2.
Extrinsic and intrinsic pathways of apoptosis mechanism. The activation of initiator caspases leads to the activation of effector caspases (the execution phase), which ultimately causes cell death. Balancing this process is the potential of cell survival pathways to promote transcription of various antiapoptotic proteins (e.g. antiapoptotic Bcl-2 family members or inhibitor of apoptosis proteins)
.
Extrinsic and intrinsic pathways of apoptosis mechanism. The activation of initiator caspases leads to the activation of effector caspases (the execution phase), which ultimately causes cell death. Balancing this process is the potential of cell survival pathways to promote transcription of various antiapoptotic proteins (e.g. antiapoptotic Bcl-2 family members or inhibitor of apoptosis proteins)
Apoptosis in OTC/T
As mentioned above, during cryopreservation, ovarian tissue is exposed to high concentrations of CPAs and then frozen in liquid nitrogen. The process of freezing and thawing can cause damage to the ovaries.5,44 Changes in physical conditions and the production of ROS during freezing cause apoptosis and activation of the caspase cell death cascade, resulting in cell destruction.4 It has been observed that during the OTC, the expression of the Fas system is induced as one of the main pathways of apoptosis in normal primary follicles.45 Another limitation of ovarian tissue transplantation is ischemia-reperfusion (IR) injury, which leads to inflammation and apoptosis due to the production of ROS and disruption of mitochondrial function.46 These changes via enhancing cytokine production and activating adhesion molecules lead to inflammatory responses and increase ovarian tissue swelling and microvascular permeability.47 Since the formation of new blood vessels after transplantation takes up to 10 days, the lack of oxygen flow to the tissue may destroy two-thirds of the ovarian tissue.24 Several studies have examined the extent of apoptosis during the cryopreservation process and it has been observed that the amount of apoptotic cells increases after vitrification of ovarian tissue.48 Apoptotic cell death in atretic follicles and decreasing in the number of primary follicles in frozen tissues have been identified.49 An experiment conducted by Adib and co-workers showed reduced normal follicles during cryopreservation compared to the control group. However, there was no significant difference in the expression of apoptosis-related genes in the two groups considerably.50 Another study showed that cryopreservation of the ovarian did not affect the morphology of the pre-antral follicle and the expression of FasL and caspase-3 apoptotic markers.51 Another experiment indicated the lower expression of the anti-apoptotic gene (BIRC5) and higher expression of pro-apoptotic genes (Fas and caspase-8) in vitrified samples compared to the control group.52
The molecular pathways of ovarian follicle survival
Apoptosis, a mechanism that can start either in ovarian germ or follicle somatic cells depending on the stage of follicle organization, mediates ovarian follicle atresia in mammals.53 The healthiest and most viable germ cells are liberated thanks to the atresia selection process. But some pathological conditions cause excessive postnatal loss of germ cells that may severely compromise fertility.54
Steroid hormones and their receptors play vital roles in follicle growth and also participate in some signaling pathways in reproductive system.55 We try to summarize some signaling pathways including WNT, insulin and Notch pathways, which are important in ovarian follicle survival and apoptosis process.56
PI3K/AKT/FOXO3 signaling
Phosphatidylinositol 3-kinase (PI3K) is a type of enzyme that phosphorylates the 3-hydroxyl group of phosphoinositides. Growth factor receptors (GFRs) activate PI3K.57 The serine/threonine kinase Akt, also known as protein kinase B, is a central node in cell signaling downstream of growth factors, cytokines, and other cellular stimuli.58 In the plasma membrane, PIP2 is converted to phosphoinositide PIP3. PI3K catalyzes this reaction.59 The result of this process is phosphorylation and activation of AKT.60 After activation, AKT travels to the nucleus and cytoplasm. It induces metabolic effects due to some targets that they can identify AKT, such as GSK3, BAD, TSC2, and FOXOs.58 The forkhead box O (FOXO) transcription factors are necessary adjusters of cellular homeostasis, OS responses and apoptosis.61 PI3K/AKT signaling pathway has a negative effect on FOXO activity. FOXO is phosphorylated by AKT, which restrain transcription.62 Absence of GFR signaling causes the conversion of PIP3 to PIP2 by PTEN. Phosphorylation of PIP3 decreases AKT activity and non-phosphorylated FOX cumulates in the nucleus.63 Activation of the PI3K/AKT pathway causes AKT to phosphorylate FOXO. Phosphorylation of FOXO causes its removal from the nucleus. Finally, the absence of FOXO leads to the inhibition of cell proliferation.64
WNT signaling
WNTs are glycoproteins that participate in the regulation of various signaling pathways. WNTs regulate signaling pathways through 3 ways. 1-β-catenin-dependent, β-catenin-independent and WNT/Ca2 + related mechanisms.65
β-Catenin is the name of a protein that is encoded by the “CTNNB1” gene in humans.66 β-catenin is an important component in the WNT-β-catenin signaling pathway. Following omission of the Ctnnb1 gene, infertility occurs in mice.67 The WNT-β-catenin signaling pathway regulates cell proliferation, differentiation and apoptosis in fetal and mature homeostasis.65 In the lack of WNT signaling, β-catenin is phosphorylated and then tolerate Ubiquitination and finally destroyed.65 It was concluded that Wnt/β-catenin signaling increases GC apoptosis. GC proliferation is inhibited by the activation of Foxo3a. Activation of Wnt/β-catenin pathway, reduces FOXO3a phosphorylation. opposed to this fact, Wnt/β-catenin signaling inhibitor IWR-1 can increase FOXO3a phosphorylation.68
Insulin signaling
Insulin is a hormone consists of 51 amino acids that is important in glucose homeostasis, cell growth and metabolism. Insulin attaches to insulin receptors (IRs) on the cell membrane.69 This connection leads to the phosphorylation of the insulin receptor (IRS). After the insulin receptor is phosphorylated, two signaling pathways, the PI3K/protein kinase B (Akt) pathway and the mitogen-activated protein kinase (MAPK) pathway are activated.70,71 Insulin signaling is very important in female fertility. Hypoinsulinemia and hyperinsulinemia has a direct effect on ovarian function.72 Insulin can regulate folliculogenesis. Insulin stimulation increases gonadotropin receptors and the sensitivity of LH to the receptors, which can increase oocyte growth.73 The simultaneous increase of insulin and FSH increases the differentiation and proliferation of theca-interstitial cells. Insulin causes steroidogenesis, the production of androgens in the human ovary and the formation of early follicles.74,75 Research shows that insulin is considered a survival factor. An increase in insulin levels cause an increase in the number of living follicles.76 FOXO3 As a nuclear transcription factor, is influenced by insulin signaling and affects the proliferation and differentiation of ovarian GCs.77
Notch signaling
Notch signaling is an important pathway in cell proliferation, cell differentiation and apoptosis.78 Notch signaling is initiated following receptor-ligand interaction. Notch receptors include Notch (Notch1-Notch4) and Notch ligands include (delta-like1, delta-like3, delta-like3, Jagged1, and Jagged2).79 The proper functioning of the vascular system is important for the ovary because it is responsible for delivering nutrients, oxygen and hormones. We conclude that the reduction of blood vessels is one of the main causes of follicular atresia. Previous researches have proven that the Notch signaling pathway is effective in angiogenesis. The function of Notch proteins and ligands was investigated in rodent ovaries and the following results were obtained. Notch 1 is expressed in the endothelium of the theca layer in the follicular phase of ovary, in endothelial cells of new vessels of corpus luteum, and in mature vessels of the theca layer in the luteal phase. Notch2, Notch3 and Jagged 2 are expressed in GCs of developing follicles.80 Notch2 is one of the most important components of the Notch pathway and has an effective presence in the theca and GCs of ovarian follicles.8.
Approaches for Ameliorating Apoptosis in OTC/T
Since ovarian tissue contains a variety of cells, including GCs, Theca cells, stromal cells, and follicles so OTC is very challenging.82 Many studies have shown that ovarian tissue develops ischemia during cryopreservation and transplantation, resulting in OS and the formation of ROS. Finally, leading to increased apoptosis and ovarian follicle death, which can affect the success rate of ovarian transplantation.83,84 Therefore, Researchers have described several approaches to minimizing apoptosis during ovarian tissue transplantation. Some successful approaches include using of the chemical compounds, antioxidants, and cell therapy (Figure 3). For example, Lee et al showed that the use of necrostatin-1 (Nec-1) during cryopreservation and transplantation has beneficial effects on ovarian tissue survival and reduces the rate of apoptosis. Nec-1 is a type of alkaloid that inhibits apoptosis by targeting receptor interacting protein-1.85 Also, it has been specified that the use of rapamycin before cryopreservation can inhibit the activation of the mammalian target of rapamycin (mTOR) pathway in thawed ovarian tissues and reduce caspase-3 expression and apoptosis.86 Lysophosphatidic acid as a signaling molecule can improve follicular development in transplanted ovaries by providing a balance between the anti- and pro-apoptotic genes in association with an increase in miR-22 expression.87 A study showed that treatment of ovarian with sphingosine 1-phosphate (S1P) block follicle atresia, decrease the expression of cleaved caspase-3, and improved their steroidogenic activity significantly.88 In addition, S1P via activating the PI3K/Akt pathway inhibits the apoptosis of GCs in response to OS induced by H2O2. S1P is a bioactive signaling molecule that plays a crucial role in inflammation, immunity, and cell proliferation.89 In other studies, it was found that the treatment of ovarian tissue with Z-VAD FMK (a broad-spectrum caspase inhibitor) during in vivo transplantation of ovarian cortex could improve primary follicular preservation and reduce apoptosis after 3 weeks of transplantation.90 In another study researchers have shown that treatment of ovarian with fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF), and autologous subcutaneous transplantation of the vitrified-thawed ovarian tissue could improve angiogenesis and significantly increased the survival rate of ovarian tissues.91 In another study, the incidence of apoptosis in ovarian tissue following cryopreservation and in vitro culture in the presence of leukemia inhibitory factor (LIF) as an anti-apoptotic agent was evaluated. This study showed that all groups treated with LIF had higher levels of progesterone and 17-β estradiol and lower Caspase-3/7 activity.92 As well as it has been shown late embryogenesis abundant proteins protect various cells from water stress and cause decreased apoptosis, improve the proliferative ability of follicles, and retain DNA/RNA integrity against cryoinjury during OTC.93,94
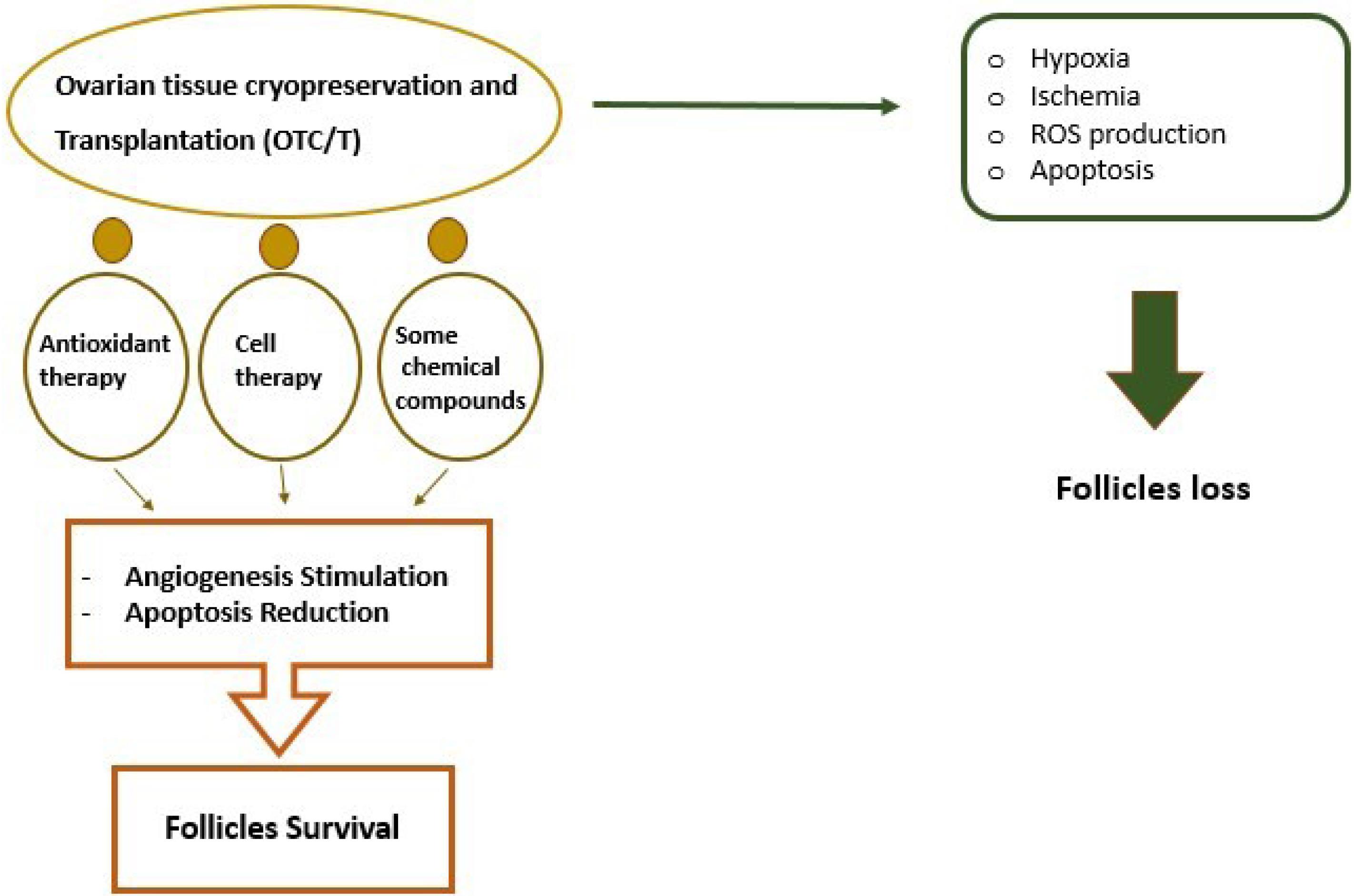
Figure 3.
Adverse effects of OTC on ovarian tissue. New approaches such as using antioxidant therapy, cell therapy, etc have emerged to increase angiogenesis and decrease apoptosis
.
Adverse effects of OTC on ovarian tissue. New approaches such as using antioxidant therapy, cell therapy, etc have emerged to increase angiogenesis and decrease apoptosis
Antioxidants
Free radicals and antioxidants actions are discussed numerously in the medicine literature. OS that is the cause of imbalance between free radicals and antioxidants capacity may be an important factor in numerous pathological conditions.95 And in reproductive biology OS is related to infertility, endometriosis, ovarian cancers, etc. Antioxidants that mainly scavenge ROS and reactive nitrogen species and also prevent their activity may play a crucial role in prevention of these adverse conditions.96
With this in mind, a wide range of compounds has been known to act as antioxidants in female reproductive system. And many studies have considered the use of antioxidants as an important strategy to reduce the production of ROS after ovarian tissue transplantation.97,98 In support of this notion, recent studies have shown that the use of Resveratrol with strong anti-inflammatory, antioxidant properties can reduce several factors associated with OS (MDA, SOD2) and inflammation (NF-κB and IL-6) during transplantation and improve the efficiency of frozen-thawed ovarian transplantation and ultimately lead to increased survival rates and decreased follicular apoptosis.99 In another experiment conducted by Kim, and co-workers, they showed that adding Klotho protein as a modulator of OS to the cryopreservation process can reduce apoptosis and DNA damage caused by ROS. Klotho is a transmembrane protein that controls OS.100 Likewise, taurine as a powerful antioxidant accelerates angiogenesis and reduces OS and apoptosis, improving follicular survival and the function of transplanted ovaries.101 Using selenium during the freezing-thawing process via upregulation of Bcl-2 and downregulation of P53 at transcription level could reduce the apoptosis induced by freezing-thawing stress.7 Also, pre-treatment of ovarian tissue with Trolox antioxidant followed by heterotopic transplantation can improve follicular quality and prevent apoptosis in stromal cells.102 High doses of the antioxidant N-acetylcysteine during autologous ovarian transplantation also lead to promote graft viability with the recovery of the estrous cycle.103 Liu et al observed that the use of melatonin reduced the production of ROS and inhibited follicular cell apoptosis. Melatonin increased expression Bcl-2 and decreased expression of Bax.104 Also, melatonin via the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway could activate anti-oxidative enzymes and inhibit OS and apoptosis during cryopreservation of ovarian tissue.105 Adding melatonin to the culture medium via reducing expression of the p53 apoptotic gene could improve the survival rate and in vitro maturation in isolated follicles from vitrified ovaries.106 Another antioxidant used in ovarian transplantation is the vitamin E improves the survival of follicles via reducing ischemia-reperfusion injury.107 Another case is erythropoietin antioxidant. A study showed that using this antioxidant can significantly reduce OS and MDA concentration.108
Cell therapy
Cell therapy is another efficient strategy to reduce follicle loss during ovarian tissue transplantation.109 Recent studies have shown that the use of stem cells in cryopreservation and transplantation of ovarian tissue increases angiogenesis, oxygenation, and follicle survival.110 Adipose tissue-derived stem cells (ASCs) have been shown to interact with cell death signal pathways and reduce ovarian follicle death after transplantation.111 ASCs have proangiogenic effects through growth factor secretion and endothelial lineage differentiation.112 In support of this notion, Manavella et al have shown that ASCs in a fibrin implant improves ovarian tissue transplantation and enhance neovascularization in the transplant site.113 These stem cells have a role in negatively modulating PI3K/Akt signaling pathways related to follicle activation and growth and positively affect the ovarian reserve by reducing follicular apoptosis.111 Two-stage transplantation with ASCs is a promising step for success in ovarian tissue transplantation due to faster re-oxygenation, increased follicle survival rate, and decreased apoptosis.114 According to many reports, mesenchymal stem cells (MSCs) derived from bone marrow, umbilical cord, amniotic fluid, placenta, etc., improve ovarian function through inhibition of follicular atresia and GC apoptosis by upregulating of the anti-Mullerian hormone.115 These cells play an essential role in supporting angiogenesis by releasing angiogenic factors and differentiating them into the endothelial cell.116 The study of Xia and colleagues that used MSCs during ovarian tissue transplantation, demonstrated that the expression levels of FGF2 and VEGF, especially the level of angiogenin in the ovary increased, and these stem cells significantly increased neo-angiogenesis, and decreased primordial follicles’ apoptotic rates.117 Mesenchymal stem cell-derived angiogenin functioned as a supportive agent for ovarian tissue and promoted angiogenesis and follicle survival.118 In another study, cord blood-derived endothelial progenitor cells were injected subcutaneously into mice, and after 1 week, vitrified/warmed ovaries were transplanted to injection sites. The results showed the survival of follicles and the formation of more blood vessels.119 According to the review of available articles, there is limited information about cell therapy during cryopreservation and ovarian transplantation. In Table 1, important and effective factors on apoptosis in OTC/T are briefly mentioned.
Table 1.
Approaches and related mechanisms for ameliorating apoptosis in ovarian tissue cryopreservation/transplantation
Preventive factors of
Post-transplantation angiogenesis
|
Apoptosis reduction mechanism
|
Examples
|
| wound angiogenesis |
Tissue damage leads to hypoxia. After injury, angiogenic factors are secreted. Peripheral blood monocytes accumulate at the site to reduce apoptosis.15 |
- |
| Antioxidants |
ROS has the ability to destroy the cell membrane. As a result, by reducing ROS, the level t of apoptosis in the follicles decreases. |
Ascorbic acid, mannitol, oxytetracycline, vitamin E10, Resveratrol and melatonin120,121 |
| Growth factors |
Growth factors are endothelial cell mitogens which induce angiogenesis. |
FGF-1, FGF-2 and VEGF120 |
| Cell therapy |
Use of stem cells can increase angiogenesis, oxygenation, and follicle survival. |
ASCs and MSCs derived from bone marrow, umbilical cord, amniotic fluid, placenta, etc. |
ROS, reactive oxygen species; FGF, fibroblast growth factor; VEGF, vascular endothelial growth factor; ASCs, adipose tissue-derived stem cells; MSCs, mesenchymal stem cells.
Preclinical studies
To analyze the preclinical stages of cryopreservation and transplantation technology, we refer to a study conducted by Suzuki et al in 2015. Seven adult female cynomolgus monkeys were used in this research. Animals were examined for 1 month in terms of infections, physical and behavioral characteristics. The monkeys were kept in separate cages and the correct functioning of their estrous cycle was vouched. The animals were anesthetized with a 2:1 mixture of ketamine and xylazine, then the ovaries of both sides were resected. The cortex tissue of the ovaries was cut into small cubes. The vitrification solution used in this study contains H199 along with 20% SSS, 5.64 M ethylene glycol, 5% (w/v) polyvinyl pyrrolidone and 0.5 M sucrose. After vitrification, the samples were immersed in liquid nitrogen. Cortical sections were placed in H199 augmented with 20% SSS and 1.61 M ethylene glycol for 10 minutes, shifted to H199 supplemented with 20% SSS and 3.22 M ethylene glycol for 10 minutes and equilibrated in VSEGP for 5 minutes. The prepared tissues were transplanted into the posterior iliac cavity, omentum, uterine serosa, and mesosalpinx as autograft transplantation. After transplantation, the monkeys were stimulated with GnRH agonist for 14 days from the first day of menstrual period. They also received pregnant mare serum gonadotrophin from day 1 to day 9. In addition, recombinant human follicle stimulating hormone was administered twice a day. On the day 10 of menstruation, the monkeys received human chorionic gonadotrophin. Oocytes were collected after 40 hours. For fertilization, sperms of male monkeys were collected and washed in 10% PVP. Then sperms were injected into mature oocytes in TALP-HEPES containing 0.3% BSA. The results of this study stated that hormonal cycles of 126 days did not show any significant difference. New blood vessels developed to supply the ovary were visualized by contrast-enhanced CT. Nine oocytes were taken from two monkeys after ovarian cortical transplantation, and six oocytes were fertilized after ICSI.122
Clinical applications
The survival rate of children with cancer has increased to 80% in most high-income countries. One of the reasons for this progress is the change in conventional treatments and lifestyle modification. Ovaries are sensitive to treatment and may be damaged during chemotherapy. To preserve the fertility of children who have survived cancer, there should be a focus on OTC/T.123 In addition to the reproductive function, the ovary also has an endocrine function. Damage to the ovary can lead to many clinical problems. Amenorrhea, menstrual disorders, decreased sexual desire, failure to develop secondary sexual characteristics, infertility and premature ovarian failure are among the complications of ovarian damage. In addition to cancer, premature ovarian failure caused by genetic, infectious and autoimmune factors can reduce fertility. Ovarian cryopreservation and transplantation technology can also be used to restore the fertility of these cases.124
Conclusion
Ovarian tissue cryopreservation and transplantation are one of the suitable options for restoring fertility in cancer survivors. However, there are many challenges in this area yet, including ROS production, ischemia and hypoxia during transplantation, leading to follicular apoptosis and ovarian damage. The usage of various chemical compounds, antioxidants, and cell therapy during the cryopreservation and transplantation process could reduce OS and apoptosis and also increase neovascularization and oxygenation. As a result, success in these areas may result in obtaining an effective method to overcome these shortcomings and preserving fertility after OTC/T.
Acknowledgements
The authors wish to appreciate the personnel of the Department of Anatomical Sciences for their help and guidance and supporting from Tabriz University of Medical Sciences.
Competing Interests
There is no competing interest to this study.
Ethical Approval
Not applicable.
References
- Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction 2003; 126(4):415-24. doi: 10.1530/rep.0.1260415 [Crossref] [ Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019; 25(6):673-93. doi: 10.1093/humupd/dmz027 [Crossref] [ Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001; 7(6):535-43. doi: 10.1093/humupd/7.6.535 [Crossref] [ Google Scholar]
- Dolmans MM, Donnez J, Cacciottola L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol Med 2021; 27(8):777-91. doi: 10.1016/j.molmed.2020.11.003 [Crossref] [ Google Scholar]
- Dittrich R, Maltaris T, Hoffmann I, Oppelt PG, Beckmann MW, Mueller A. Fertility preservation in cancer patients. Minerva Ginecol 2010; 62(1):63-80. [ Google Scholar]
- Arian SE, Goodman L, Flyckt RL, Falcone T. Ovarian transposition: a surgical option for fertility preservation. Fertil Steril 2017; 107(4):e15. doi: 10.1016/j.fertnstert.2017.01.010 [Crossref] [ Google Scholar]
- Moawad NS, Santamaria E, Rhoton-Vlasak A, Lightsey JL. Laparoscopic ovarian transposition before pelvic cancer treatment: ovarian function and fertility preservation. J Minim Invasive Gynecol 2017; 24(1):28-35. doi: 10.1016/j.jmig.2016.08.831 [Crossref] [ Google Scholar]
- Morice P, Juncker L, Rey A, El-Hassan J, Haie-Meder C, Castaigne D. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil Steril 2000; 74(4):743-8. doi: 10.1016/s0015-0282(00)01500-4 [Crossref] [ Google Scholar]
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys 2009; 73(5):1304-12. doi: 10.1016/j.ijrobp.2008.12.016 [Crossref] [ Google Scholar]
- Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab 2006; 91(10):3885-90. doi: 10.1210/jc.2006-0962 [Crossref] [ Google Scholar]
- Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril 2013; 99(6):1496-502. doi: 10.1016/j.fertnstert.2013.03.020 [Crossref] [ Google Scholar]
- El-Danasouri I, Selman H. Successful pregnancies and deliveries after a simple vitrification protocol for day 3 human embryos. Fertil Steril 2001; 76(2):400-2. doi: 10.1016/s0015-0282(01)01907-0 [Crossref] [ Google Scholar]
- Cao B, Qin J, Pan B, Qazi IH, Ye J, Fang Y. Oxidative stress and oocyte cryopreservation: recent advances in mitigation strategies involving antioxidants. Cells 2022; 11(22):3573. doi: 10.3390/cells11223573 [Crossref] [ Google Scholar]
- Tao T, Del Valle A. Human oocyte and ovarian tissue cryopreservation and its application. J Assist Reprod Genet 2008; 25(7):287-96. doi: 10.1007/s10815-008-9236-z [Crossref] [ Google Scholar]
- Bahroudi Z, Rezaei Zarnaghi M, Izadpanah M, Abedelahi A, Niknafs B, Tayefi Nasrabadi H. Review of ovarian tissue cryopreservation techniques for fertility preservation. J Gynecol Obstet Hum Reprod 2022; 51(2):102290. doi: 10.1016/j.jogoh.2021.102290 [Crossref] [ Google Scholar]
- Andersen CY, Bollerup AC, Kristensen SG. Defining quality assurance and quality control measures in connection with ovarian tissue cryopreservation and transplantation: a call to action. Hum Reprod 2018; 33(7):1201-4. doi: 10.1093/humrep/dey105 [Crossref] [ Google Scholar]
- Shirazi Tehrani A, Mazoochi T, Akhavan Taheri M, Aghadavood E, Salehnia M. The effects of ovarian encapsulation on morphology and expression of apoptosis-related genes in vitrified mouse ovary. J Reprod Infertil 2021; 22(1):23-31. doi: 10.18502/jri.v22i1.4992 [Crossref] [ Google Scholar]
- Dolmans MM, Donnez J. Indications for fertility preservation in women from malignant diseases to benign conditions to age-related fertility decline. Minerva Ginecol 2018; 70(4):402-7. doi: 10.23736/s0026-4784.18.04232-6 [Crossref] [ Google Scholar]
- Andersen CY, Kristensen SG. Novel use of the ovarian follicular pool to postpone menopause and delay osteoporosis. Reprod Biomed Online 2015; 31(2):128-31. doi: 10.1016/j.rbmo.2015.05.002 [Crossref] [ Google Scholar]
- Kim SS. Assessment of long-term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10-year longitudinal follow-up study. J Assist Reprod Genet 2012; 29(6):489-93. doi: 10.1007/s10815-012-9757-3 [Crossref] [ Google Scholar]
- Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med 2000; 342(25):1919. doi: 10.1056/nejm200006223422516 [Crossref] [ Google Scholar]
- Donfack NJ, Alves KA, Araújo VR, Cordova A, Figueiredo JR, Smitz J. Expectations and limitations of ovarian tissue transplantation. Zygote 2017; 25(4):391-403. doi: 10.1017/s0967199417000338 [Crossref] [ Google Scholar]
- Ballestín A, Casado JG, Abellán E, Vela FJ, Álvarez V, Usón A. Ischemia-reperfusion injury in a rat microvascular skin free flap model: a histological, genetic, and blood flow study. PLoS One 2018; 13(12):e0209624. doi: 10.1371/journal.pone.0209624 [Crossref] [ Google Scholar]
- Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril 2010; 93(5):1676-85. doi: 10.1016/j.fertnstert.2009.04.048 [Crossref] [ Google Scholar]
- Lee J, Kong HS, Kim EJ, Youm HW, Lee JR, Suh CS. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod 2016; 31(8):1827-37. doi: 10.1093/humrep/dew144 [Crossref] [ Google Scholar]
- Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril 2000; 74(1):122-9. doi: 10.1016/s0015-0282(00)00548-3 [Crossref] [ Google Scholar]
- Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology 2003; 124(2):494-503. doi: 10.1053/gast.2003.50059 [Crossref] [ Google Scholar]
- Gualtieri R, Kalthur G, Barbato V, Di Nardo M, Adiga SK, Talevi R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants (Basel) 2021; 10(3):337. doi: 10.3390/antiox10030337 [Crossref] [ Google Scholar]
- Zhou Y, Liu H, Zhao N, Wang Z, Michael MZ, Xie N. Multiplexed imaging detection of live cell intracellular changes in early apoptosis with aggregation-induced emission fluorogens. Sci China Chem 2018; 61(8):892-7. doi: 10.1007/s11426-018-9287-x [Crossref] [ Google Scholar]
- Oberst A, Bender C, Green DR. Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ 2008; 15(7):1139-46. doi: 10.1038/cdd.2008.65 [Crossref] [ Google Scholar]
- Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY) 2012; 4(5):330-49. doi: 10.18632/aging.100459 [Crossref] [ Google Scholar]
- Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 2012; 45(6):487-98. doi: 10.1111/j.1365-2184.2012.00845.x [Crossref] [ Google Scholar]
- Yadav PK, Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN. Germ cell depletion from mammalian ovary: possible involvement of apoptosis and autophagy. J Biomed Sci 2018; 25(1):36. doi: 10.1186/s12929-018-0438-0 [Crossref] [ Google Scholar]
- Sun YC, Sun XF, Dyce PW, Shen W, Chen H. The role of germ cell loss during primordial follicle assembly: a review of current advances. Int J Biol Sci 2017; 13(4):449-57. doi: 10.7150/ijbs.18836 [Crossref] [ Google Scholar]
- Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol 2017; 217(2):129-40. doi: 10.1016/j.ajog.2017.02.027 [Crossref] [ Google Scholar]
- Flemming W. Uber die bildung von richtungsfiguren in saugethiereiern beim untergang graaf’scher follikel. Arch Anat EntwGesh. 1885:221-4.
- Vaskivuo TE, Tapanainen JS. Apoptosis in the human ovary. Reprod Biomed Online 2003; 6(1):24-35. doi: 10.1016/s1472-6483(10)62052-4 [Crossref] [ Google Scholar]
- Jancar N, Kopitar AN, Ihan A, Virant Klun I, Bokal EV. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet 2007; 24(2-3):91-7. doi: 10.1007/s10815-006-9103-8 [Crossref] [ Google Scholar]
- Yu YS, Sui HS, Han ZB, Li W, Luo MJ, Tan JH. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res 2004; 14(4):341-6. doi: 10.1038/sj.cr.7290234 [Crossref] [ Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update 2005; 11(2):162-77. doi: 10.1093/humupd/dmi001 [Crossref] [ Google Scholar]
- Zhang J, Xu Y, Liu H, Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol 2019; 17(1):9. doi: 10.1186/s12958-018-0450-y [Crossref] [ Google Scholar]
- Li Q, Du X, Liu L, Liu H, Pan Z, Li Q. Upregulation of miR-146b promotes porcine ovarian granulosa cell apoptosis by attenuating CYP19A1. Domest Anim Endocrinol 2021; 74:106509. doi: 10.1016/j.domaniend.2020.106509 [Crossref] [ Google Scholar]
- Liu J, Li X, Yao Y, Li Q, Pan Z, Li Q. miR-1275 controls granulosa cell apoptosis and estradiol synthesis by impairing LRH-1/CYP19A1 axis. Biochim Biophys Acta Gene Regul Mech 2018; 1861(3):246-57. doi: 10.1016/j.bbagrm.2018.01.009 [Crossref] [ Google Scholar]
- Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod Biomed Online 2004; 9(2):164-70. doi: 10.1016/s1472-6483(10)62125-6 [Crossref] [ Google Scholar]
- Xiao Z, Wang Y, Li L, Li SW. Cryopreservation of the human ovarian tissue induces the expression of Fas system in morphologically normal primordial follicles. Cryo Letters 2010; 31(2):112-9. [ Google Scholar]
- Juránek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species--cause or consequence of tissue injury?. Gen Physiol Biophys 2005; 24(3):263-78. [ Google Scholar]
- Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 2010; 35(9):505-13. doi: 10.1016/j.tibs.2010.04.002 [Crossref] [ Google Scholar]
- Rahimi G, Isachenko V, Todorov P, Tawadros S, Mallmann P, Nawaroth F. Apoptosis in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Cryo Letters 2009; 30(4):300-9. [ Google Scholar]
- Bedaiwy MA, Hussein MR. Histological evaluation and in situ localization of apoptosis in fresh and cryopreserved ovarian tissue. Middle East Fertil Soc J 2004; 9(2):163-70. [ Google Scholar]
- Adib S, Valojerdi MR, Alikhani M. Evaluation of apoptotic markers and tissue histology indicate a slight advantage of slow freezing method over vitrification for sheep ovarian tissues. Cryo Letters 2018; 39(5):313-21. [ Google Scholar]
- Wiweko B, Soebijanto S, Boediono A, Mansyur M, Siregar NC, Suryandari DA. Survival of isolated human preantral follicles after vitrification: analyses of morphology and Fas ligand and caspase-3 mRNA expression. Clin Exp Reprod Med 2019; 46(4):152-65. doi: 10.5653/cerm.2019.00143 [Crossref] [ Google Scholar]
- Abdollahi M, Salehnia M, Salehpour S, Ghorbanmehr N. Human ovarian tissue vitrification/warming has minor effect on the expression of apoptosis-related genes. Iran Biomed J 2013; 17(4):179-86. doi: 10.6091/ibj.1243.2013 [Crossref] [ Google Scholar]
- Prasasya RD, Mayo KE. Regulation of follicle formation and development by ovarian signaling pathways. In: Leung PCK, Adashi EY, eds. The Ovary. 3rd ed. Academic Press; 2019. p. 23-49. 10.1016/b978-0-12-813209-8.00002-9.
- Johnson AL. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci 2003; 78(3-4):185-201. doi: 10.1016/s0378-4320(03)00090-3 [Crossref] [ Google Scholar]
- Shi J, Liu C, Chen M, Yan J, Wang C, Zuo Z. The interference effects of bisphenol A on the synthesis of steroid hormones in human ovarian granulosa cells. Environ Toxicol 2021; 36(4):665-74. doi: 10.1002/tox.23070 [Crossref] [ Google Scholar]
- Hou HW, Xue P, Wang Y, Li YK. Liraglutide regulates proliferation, differentiation, and apoptosis of preosteoblasts through a signaling network of Notch/Wnt/Hedgehog signaling pathways. Eur Rev Med Pharmacol Sci 2020; 24(23):12408-22. doi: 10.26355/eurrev_202012_24037 [Crossref] [ Google Scholar]
- Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell 2017; 170(4):605-35. doi: 10.1016/j.cell.2017.07.029 [Crossref] [ Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007; 129(7):1261-74. doi: 10.1016/j.cell.2007.06.009 [Crossref] [ Google Scholar]
- Katan M, Cockcroft S. Phosphatidylinositol(4,5)bisphosphate: diverse functions at the plasma membrane. Essays Biochem 2020; 64(3):513-31. doi: 10.1042/ebc20200041 [Crossref] [ Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008; 321(1):197-204. doi: 10.1016/j.ydbio.2008.06.017 [Crossref] [ Google Scholar]
- Tsuchiya K, Ogawa Y. Forkhead box class O family member proteins: the biology and pathophysiological roles in diabetes. J Diabetes Investig 2017; 8(6):726-34. doi: 10.1111/jdi.12651 [Crossref] [ Google Scholar]
- Zhang M, Zhang X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch Dermatol Res 2019; 311(2):83-91. doi: 10.1007/s00403-018-1879-8 [Crossref] [ Google Scholar]
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 2013; 14(2):83-97. doi: 10.1038/nrm3507 [Crossref] [ Google Scholar]
- Stefanetti RJ, Voisin S, Russell A, Lamon S. Recent advances in understanding the role of FOXO3. F1000Res 2018; 7:F1000 Faculty Rev-1372. doi: 10.12688/f1000research.15258.1 [Crossref] [ Google Scholar]
- Hernandez Gifford JA. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 2015; 150(4):R137-48. doi: 10.1530/rep-14-0685 [Crossref] [ Google Scholar]
- Monga SP. β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015; 148(7):1294-310. doi: 10.1053/j.gastro.2015.02.056 [Crossref] [ Google Scholar]
- Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH. Conditional deletion of beta-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod 2009; 80(6):1282-92. doi: 10.1095/biolreprod.108.072280 [Crossref] [ Google Scholar]
- Li L, Ji SY, Yang JL, Li XX, Zhang J, Zhang Y. Wnt/β-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Mol Cell Endocrinol 2014; 382(2):915-25. doi: 10.1016/j.mce.2013.11.007 [Crossref] [ Google Scholar]
- Lewis GF, Brubaker PL. The discovery of insulin revisited: lessons for the modern era. J Clin Invest 2021; 131(1):e142239. doi: 10.1172/jci142239 [Crossref] [ Google Scholar]
- Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 2018; 19(1):31-44. doi: 10.1038/nrm.2017.89 [Crossref] [ Google Scholar]
- Saltiel AR. Insulin signaling in health and disease. J Clin Invest 2021; 131(1):e142241. doi: 10.1172/jci142241 [Crossref] [ Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33(6):981-1030. doi: 10.1210/er.2011-1034 [Crossref] [ Google Scholar]
- Das D, Arur S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol Reprod Dev 2017; 84(6):444-59. doi: 10.1002/mrd.22806 [Crossref] [ Google Scholar]
- Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes 2021; 12(5):616-29. doi: 10.4239/wjd.v12.i5.616 [Crossref] [ Google Scholar]
- de Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum Reprod Update 2021; 27(4):771-96. doi: 10.1093/humupd/dmab004 [Crossref] [ Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010; 140(5):685-97. doi: 10.1530/rep-10-0284 [Crossref] [ Google Scholar]
- Tan M, Cheng Y, Zhong X, Yang D, Jiang S, Ye Y. LNK promotes granulosa cell apoptosis in PCOS via negatively regulating insulin-stimulated AKT-FOXO3 pathway. Aging (Albany NY) 2021; 13(3):4617-33. doi: 10.18632/aging.202421 [Crossref] [ Google Scholar]
- Liu YX, Zhang Y, Li YY, Liu XM, Wang XX, Zhang CL. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front Biosci (Landmark Ed) 2019; 24(5):983-93. doi: 10.2741/4763 [Crossref] [ Google Scholar]
- Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol 2014; 28(4):499-511. doi: 10.1210/me.2013-1288 [Crossref] [ Google Scholar]
- Xie Q, Cheng Z, Chen X, Lobe CG, Liu J. The role of Notch signalling in ovarian angiogenesis. J Ovarian Res 2017; 10(1):13. doi: 10.1186/s13048-017-0308-5 [Crossref] [ Google Scholar]
- Jing J, Jiang X, Chen J, Yao X, Zhao M, Li P. Notch signaling pathway promotes the development of ovine ovarian follicular granulosa cells. Anim Reprod Sci 2017; 181:69-78. doi: 10.1016/j.anireprosci.2017.03.017 [Crossref] [ Google Scholar]
- Hossay C, Donnez J, Dolmans MM. Whole ovary cryopreservation and transplantation: a systematic review of challenges and research developments in animal experiments and humans. J Clin Med 2020; 9(10):3196. doi: 10.3390/jcm9103196 [Crossref] [ Google Scholar]
- Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod 2002; 17(3):605-11. doi: 10.1093/humrep/17.3.605 [Crossref] [ Google Scholar]
- Xie S, Zhang X, Chen W, Xie C, Chen W, Cheng P. Developmental status: impact of short-term ischemia on follicular survival of whole ovarian transplantation in a rabbit model. PLoS One 2015; 10(8):e0135049. doi: 10.1371/journal.pone.0135049 [Crossref] [ Google Scholar]
- Lee JR, Youm HW, Kim SK, Jee BC, Suh CS, Kim SH. Effect of necrostatin on mouse ovarian cryopreservation and transplantation. Eur J Obstet Gynecol Reprod Biol 2014; 178:16-20. doi: 10.1016/j.ejogrb.2014.04.040 [Crossref] [ Google Scholar]
- Liu W, Zhang J, Wang L, Liang S, Xu B, Ying X. The protective effects of rapamycin pretreatment on ovarian damage during ovarian tissue cryopreservation and transplantation. Biochem Biophys Res Commun 2021; 534:780-6. doi: 10.1016/j.bbrc.2020.10.110 [Crossref] [ Google Scholar]
- Dehghan M, Shahbazi SH, Salehnia M. Lysophosphatidic acid alters the expression of apoptosis related genes and miR-22 in cultured and autotransplanted ovaries. Cell J 2021; 23(5):584-92. doi: 10.22074/cellj.2021.7303 [Crossref] [ Google Scholar]
- Guzel Y, Bildik G, Oktem O. Sphingosine-1-phosphate protects human ovarian follicles from apoptosis in vitro. Eur J Obstet Gynecol Reprod Biol 2018; 222:19-24. doi: 10.1016/j.ejogrb.2018.01.001 [Crossref] [ Google Scholar]
- Nakahara T, Iwase A, Nakamura T, Kondo M, Bayasula, Kobayashi H, et al. Sphingosine-1-phosphate inhibits H2O2-induced granulosa cell apoptosis via the PI3K/Akt signaling pathway. Fertil Steril 2012;98(4):1001-8.e1. 10.1016/j.fertnstert.2012.06.008.
- Fransolet M, Noël L, Henry L, Labied S, Blacher S, Nisolle M. Evaluation of Z-VAD-FMK as an anti-apoptotic drug to prevent granulosa cell apoptosis and follicular death after human ovarian tissue transplantation. J Assist Reprod Genet 2019; 36(2):349-59. doi: 10.1007/s10815-018-1353-8 [Crossref] [ Google Scholar]
- Li SH, Hwu YM, Lu CH, Chang HH, Hsieh CE, Lee RK. VEGF and FGF2 improve revascularization, survival, and oocyte quality of cryopreserved, subcutaneously-transplanted mouse ovarian tissues. Int J Mol Sci 2016; 17(8):1237. doi: 10.3390/ijms17081237 [Crossref] [ Google Scholar]
- Abdollahi M, Salehnia M, Salehpour S, Pour-Beiranvand S. Analysis of apoptosis in cultured human vitrified ovarian tissue in the presence of leukemia inhibitory factor. J Reprod Infertil 2018; 19(4):193-202. [ Google Scholar]
- Rendón-Luna DF, Arroyo-Mosso IA, De Luna-Valenciano H, Campos F, Segovia L, Saab-Rincón G. Alternative conformations of a group 4 late embryogenesis abundant protein associated to its in vitro protective activity. Sci Rep 2024; 14(1):2770. doi: 10.1038/s41598-024-53295-7 [Crossref] [ Google Scholar]
- Shih MD, Hoekstra FA, Hsing YI. Late embryogenesis abundant proteins. Adv Bot Res 2008; 48:211-55. doi: 10.1016/s0065-2296(08)00404-7 [Crossref] [ Google Scholar]
- Sisein EA. Biochemistry of free radicals and antioxidants. Sch Acad J Biosci 2014; 2(2):110-8. [ Google Scholar]
- Sekhon L, Gupta S, Kim Y, Agarwal A. Female infertility and antioxidants. Curr Womens Health Rev 2010; 6(2):84-95. doi: 10.2174/157340410791321381 [Crossref] [ Google Scholar]
- Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M. Importance of melatonin in assisted reproductive technology and ovarian aging. Int J Mol Sci 2020; 21(3):1135. doi: 10.3390/ijms21031135 [Crossref] [ Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3:28. doi: 10.1186/1477-7827-3-28 [Crossref] [ Google Scholar]
- Wang D, Geng M, Gan D, Han G, Gao G, Xing A. Effect of resveratrol on mouse ovarian vitrification and transplantation. Reprod Biol Endocrinol 2021; 19(1):54. doi: 10.1186/s12958-021-00735-y [Crossref] [ Google Scholar]
- Kim B, Yoon H, Kim T, Lee S. Role of klotho as a modulator of oxidative stress associated with ovarian tissue cryopreservation. Int J Mol Sci 2021; 22(24):13547. doi: 10.3390/ijms222413547 [Crossref] [ Google Scholar]
- Ahmadi S, Soleimani Mehranjani M. Taurine improves follicular survival and function of mice ovarian grafts through increasing CD31 and GDF9 expression and reducing oxidative stress and apoptosis. Eur J Pharmacol 2021; 903:174134. doi: 10.1016/j.ejphar.2021.174134 [Crossref] [ Google Scholar]
- Scalercio SR, Amorim CA, Brito DC, Percário S, Oskam IC, Domingues SF. Trolox enhances follicular survival after ovarian tissue autograft in squirrel monkey (Saimiri collinsi). Reprod Fertil Dev 2016; 28(11):1854-64. doi: 10.1071/rd14454 [Crossref] [ Google Scholar]
- de Amorim EM, Damous LL, Durando MC, Saraiva MV, Koike MK, de Souza Montero EF. N-acetylcysteine improves morphologic and functional aspects of ovarian grafts in rats. Acta Cir Bras 2014; 29 Suppl 3:22-7. doi: 10.1590/s0102-86502014001700005 [Crossref] [ Google Scholar]
- Liu XC, Sun TC, Li HY, Si LN, Wei M, Chen ZH. Antioxidative effect of melatonin on cryopreserved ovarian tissue in mice. Cryobiology 2020; 96:99-105. doi: 10.1016/j.cryobiol.2020.07.010 [Crossref] [ Google Scholar]
- Sun TC, Liu XC, Yang SH, Song LL, Zhou SJ, Deng SL. Melatonin inhibits oxidative stress and apoptosis in cryopreserved ovarian tissues via Nrf2/HO-1 signaling pathway. Front Mol Biosci 2020; 7:163. doi: 10.3389/fmolb.2020.00163 [Crossref] [ Google Scholar]
- Mazoochi T, Khamechian T, Ehteram M, Haddad Kashani H. The effect of melatonin on expression of p53 and ovarian preantral follicle development isolated from vitrified ovary. Comparative Clinical Pathology 2018; 27(1):83-8. doi: 10.1007/s00580-017-2555-7 [Crossref] [ Google Scholar]
- Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil 1998; 114(2):341-6. doi: 10.1530/jrf.0.1140341 [Crossref] [ Google Scholar]
- Mahmoodi M, Soleimani Mehranjani M, Shariatzadeh SM, Eimani H, Shahverdi A. Effects of erythropoietin on ischemia, follicular survival, and ovarian function in ovarian grafts. Reproduction 2014; 147(5):733-41. doi: 10.1530/rep-13-0379 [Crossref] [ Google Scholar]
- Cacciottola L, Nguyen TYT, Chiti MC, Camboni A, Amorim CA, Donnez J. Long-term advantages of ovarian reserve maintenance and follicle development using adipose tissue-derived stem cells in ovarian tissue transplantation. J Clin Med 2020; 9(9):2980. doi: 10.3390/jcm9092980 [Crossref] [ Google Scholar]
- Kim JM, Kim S, Lee S. Role of stem cells in the ovarian tissue cryopreservation and transplantation for fertility preservation. Int J Mol Sci 2021; 22(22):12482. doi: 10.3390/ijms222212482 [Crossref] [ Google Scholar]
- Cacciottola L, Courtoy GE, Nguyen TY, Hossay C, Donnez J, Dolmans MM. Adipose tissue-derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation. J Assist Reprod Genet 2021; 38(1):151-61. doi: 10.1007/s10815-020-02005-z [Crossref] [ Google Scholar]
- Manavella DD, Cacciottola L, Payen VL, Amorim CA, Donnez J, Dolmans MM. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol Hum Reprod 2019; 25(4):184-93. doi: 10.1093/molehr/gaz008 [Crossref] [ Google Scholar]
- Manavella DD, Cacciottola L, Desmet CM, Jordan BF, Donnez J, Amorim CA. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: a potential way to improve ovarian tissue transplantation. Hum Reprod 2018; 33(2):270-9. doi: 10.1093/humrep/dex374 [Crossref] [ Google Scholar]
- Manavella DD, Cacciottola L, Pommé S, Desmet CM, Jordan BF, Donnez J. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum Reprod 2018; 33(6):1107-16. doi: 10.1093/humrep/dey080 [Crossref] [ Google Scholar]
- Yoon SY. Mesenchymal stem cells for restoration of ovarian function. Clin Exp Reprod Med 2019; 46(1):1-7. doi: 10.5653/cerm.2019.46.1.1 [Crossref] [ Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009; 4(3):206-16. doi: 10.1016/j.stem.2009.02.001 [Crossref] [ Google Scholar]
- Xia X, Yin T, Yan J, Yan L, Jin C, Lu C. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant 2015; 24(10):1999-2010. doi: 10.3727/096368914x685267 [Crossref] [ Google Scholar]
- Zhang Y, Xia X, Yan J, Yan L, Lu C, Zhu X. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod Biol Endocrinol 2017; 15(1):18. doi: 10.1186/s12958-017-0235-8 [Crossref] [ Google Scholar]
- Cha SK, Shin DH, Kim BY, Yoon SY, Yoon TK, Lee WS. Effect of human endothelial progenitor cell (EPC)- or mouse vascular endothelial growth factor-derived vessel formation on the survival of vitrified/warmed mouse ovarian grafts. Reprod Sci 2014; 21(7):859-68. doi: 10.1177/1933719113518983 [Crossref] [ Google Scholar]
- Bedaiwy MA, Falcone T. Whole ovary transplantation. Clin Obstet Gynecol 2010; 53(4):797-803. doi: 10.1097/GRF.0b013e3181f97c94 [Crossref] [ Google Scholar]
- Izadpanah M, Rahmani Del Bakhshayesh A, Bahroudi Z, Majdi Seghinsara A, Beheshti R, Mahdipour M. Melatonin and endothelial cell-loaded alginate-fibrin hydrogel promoted angiogenesis in rat cryopreserved/thawed ovaries transplanted to the heterotopic sites. J Biol Eng 2023; 17(1):23. doi: 10.1186/s13036-023-00343-x [Crossref] [ Google Scholar]
- Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod 2012; 27(8):2420-9. doi: 10.1093/humrep/des178 [Crossref] [ Google Scholar]
- Lam CG, Howard SC, Bouffet E, Pritchard-Jones K. Science and health for all children with cancer. Science 2019; 363(6432):1182-6. doi: 10.1126/science.aaw4892 [Crossref] [ Google Scholar]
- Kim SS, Battaglia DE, Soules MR. The future of human ovarian cryopreservation and transplantation: fertility and beyond. Fertil Steril 2001; 75(6):1049-56. doi: 10.1016/s0015-0282(01)01790-3 [Crossref] [ Google Scholar]