ImmunoAnalysis. 4:1.
doi: 10.34172/ia.2024.01
Original Article
Docosahexaenoic Acid (DHA) Down-regulates Cellular, and Exosomal Overexpressed Mammalian Target of Rapamycin (mTOR) in Breast Cancer Cells Under Hypoxic Condition
Sepideh Maralbashi Conceptualization, Investigation, Writing – original draft, 1, 2 
Farhad Salari Formal analysis, Validation, Visualization, 2
Cynthia Aslan Investigation, Methodology, Writing – review & editing, 3
Houman Kahroba Investigation, Methodology, Writing – review & editing, 4, 5
Milad Asadi Data curation, Methodology, Software, 6
Zahra Asghari Investigation, Writing – review & editing, 6
Tohid Kazemi Conceptualization, Funding acquisition, Supervision, Validation, 6, * 
Author information:
1Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Department of Immunology, Faculty of Medicine, Kermanshah University of Medical Science, Kermanshah, Iran
3Applied Drug Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Toxicogenomics, GROW School for Oncology and Developmental Biology, Maastricht University, the Netherlands
5Centre for Environmental Sciences, Hasselt University, Hasselt, Belgium
6Immunology Research Center, Tabriz University of Medical Science, Tabriz, Iran
Abstract
Background:
The omega-3 long-chain polyunsaturated fatty acid, docosahexaenoic acid (DHA), shows anti-proliferative effects in cancer cell lines and animal models. The mammalian target of rapamycin (mTOR) is one of the regulators for the proliferation and survival of cancer cells. This study focused on the effect of DHA on cellular and exosomal expression of mTOR and related tumor-suppressor microRNAs (miRs) in triple-positive (BT-474) and triple-negative (MDA-MB-231) breast cancer (BC) cell lines.
Methods:
BT-474 and MDA-MB-231 cells were treated with 100 μM DHA under hypoxic and normoxic conditions for 24 hours. The exosomes were isolated by ultracentrifuge and determined by electron microscopy and CD9, CD63, and CD81 immunoblotting. cDNAs from cellular and exosomal total RNA were used for evaluation of the expression of mTOR and related tumor-suppressor miRs, miR-101 and miR-214, by quantitative real-time PCR.
Results:
We demonstrated that DHA significantly decreased cellular and exosomal expression of mTOR in both normoxic and hypoxic conditions for both cell lines. Consistently, DHA caused significantly increased expression of miR-214 in all treated groups. However, altered expression of miR-101 showed different patterns in cells and exosomes.
Conclusion:
According to the beneficial effect of DHA in decreasing the expression of a master regulator for the proliferation of cancer cells, mTOR, in part by increased expression of miR-214, it could be used as a supplementary therapy in BC treatment. Also, miRNA replacement therapy would be useful by suppressing the expression of mTOR in BC treatment.
Keywords: DHA, Breast cancer, Exosome, mTOR, miR-101, miR-214
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was funded by grants from the Immunology Research Center, Tabriz University of Medical Science, Tabriz, Iran (grant number: 68168).
Introduction
Breast cancer (BC) allocates the largest cancer group in females, with more than 2 million new diagnosis cases in 2018. It is also the main agent of cancer-related mortality in women around the world, above 500 000 passing away reports annually.1 Despite recent developments in metastases of node-negative or positive and drug resistance, there are obstacles in the treatment of BC.2 Hence, novel strategies and therapeutic approaches are required to dissolve these clinical challenges.
Currently, studies have shown that nutritional interventions could be able to diminish the risk of BC.3 Among the dietary components, n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs) especially docosahexaenoic acid (DHA) is a hopeful candidate for the prevention of BC.2 It has many useful therapeutic features such as anti-inflammatory and anti-cancer effects. Compared to anti-cancer drugs, DHA presents a non-toxic and safe agent to suppress the growth of neoplasm.4 Earlier in vivo and in vitro research in the field of mammary carcinogenesis showed that DHA is a stronger chemo-preventive agent than other omega-3 fatty acids.5
There is evidence that n-3 LCPUFAs could be able to reduce cell growth of BC cells by suppression of the mammalian target of rapamycin (mTOR) which is a basic member of a signaling pathway in the pathogenesis of BC.6,7 This master regulator protein has a vital role in the adjustment of growth, metabolism, cell survival, and protein synthesis, where approximately 60% of the carcinomas demonstrate different alterations that cause to increase in the activation of this pathway.8,9
Another important factor in cancer progression that has been discovered in recent years is exosomes. Cancer cells continuously produce exosomes, then release, and utilize them to increase the migration, proliferation, and angiogenesis of cancerous cells.10 Exosomes have variate contents including lipids (cholesterol, ceramide, phosphatidylserine, etc), proteins (tetraspanin, adhesion molecules, MHC class II, etc), and nucleic acids (mRNA, DNA, miRNA, etc). These cargos are transferred to target cells, activate particular intracellular cascades and influence the gene expression profile of the target cells.10,11 One of the endogenous and small noncoding RNAs (~20–25 nt) are miRNAs which have a significant role in tumor progression as tumor suppressors or oncogenes.11
The major objectives of this study were to investigate the effect of DHA on the expression of the mTOR gene and miR-101, and miR-214 both in MDA-MB-231 (as a metastatic triple-negative BC cell line) and BT-474 cells (as a non-metastatic triple-positive cell line) and also in their secreted exosomes under hypoxic and normoxic conditions.
Materials and Methods
Cell culture and treatments
Two human BC cell lines (BT-474 and MDA-MB-231) used in this study were purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). Cells were cultured in complete Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco Inc., El Paso, TX) supplemented by 10% of fetal bovine serum (Gibco Inc.), 100 unit/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C in a 95% humidified incubator with 5% CO2. Cells with 80% confluency were treated with 100µM DHA (Sigma-Aldrich) and/or 25μM CoCl2 for 24 hours. Cells treated with vehicle buffer were considered as control cells.
Exosome isolation
Up on reaching cells to 80% confluency (1 × 106 cells/mL), the supernatant was removed and cells were washed by RPMI 1640 medium. Then, cell culture was proceeded by RPMI 1640 medium supplemented with the 5% exosome-free FBS for 24 hours. Subsequently, the supernatants were subjected to centrifugation at 500 × g for 30 minutes (4 ˚C) (Hettich Rotanta 46R, German) to eliminate cells and debris. The isolation process was followed by 30 000 × g centrifugation for 2 hours (4 ˚C) to eliminate the micro-vesicles and filtration by membrane filter (0.22 µm) and then ultracentrifugation at 110 000 × g (4˚C) for 2 hours (TLA-100.3, Beckman Coulter Life Sciences, Indiana, US). The exosome pellet was added in 1:20 PBS then sonicated three times for 30 seconds and kept at -80 °C for further experiments.
Transmitting electron microscope
Exosome morphology was assessed by transmission electron microscope (TEM). A droplet of exosome-containing solution was used to fix the exosomes on a 300 mesh copper grid at ambient temperature for 5 minutes. Then, negative staining was applied to the exosome with 2% (wt/v) uranyl acetate solution (TAAB, England) for 5 minutes and visualized by LEO 906 Zeiss instrument with 80 kV quickening voltage.12
Western blot analysis
Exosome lysate was prepared by using 250 µL of protein lysis buffer (RIPA, Sigma-Aldrich). Before loading on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the exosomal proteins were incubated with β-mercaptoethanol containing loading buffer for 10 minutes at 70 ˚C. The extracted proteins were transferred onto the polyvinylidene difluoride membrane using the wet transfer system (Mini Trans‐Blot Cell, Bio‐Rad, Hercules, CA) in 120 M-voltage at 1.30 hours. Then, the membrane was blocked with the TBST buffer containing 2% non-fat powdered milk at room temperature for 1.15 hours. Incubation with the anti-exosomal markers monoclonal primary antibodies (SANTA CRUZ, California, USA) using the 1:1000 dilution was done at 4 °C for 18 hours. The membrane was washed three times for 15 minutes each time with TBST buffer. The membrane was incubated with anti-rabbit secondary antibody (1:1000) for all primary antibodies for 1.15 hours at room temperature. At the end of this step, the membrane was washed three times with TBST buffer for 15 minutes each time. Anti-GAPDH monoclonal antibody (Santa Cruz Biotechnology) was used as a positive control. Detection of the target proteins was performed by the Super-Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and the Western blot imaging system (Sabz Co, Iran). Finally, the ImageJ software (National Institutes of Health, Maryland) was used for the analysis of the intensity of protein bands.
Quantitative real-time PCR
Cell lines and isolated exosomes were used for RNA extraction by the TRIzol method (RiboEX, GeneAll Biotechnology, Korea) recommended by the manufacturer. RNA concentration was evaluated by Nano-Drop Spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA) and then kept at -80 °C for future experiments. Extracted RNA samples were applied to assess the extraction qualitatively by electrophoresis on an agarose gel to observe 18s and 28s ribosomal RNA bands. miRNAs were reverse transcribed by Universal cDNA Synthesis Kit II (Exiqon, Vedbaek, Denmark) based on the manufacturer’s instruction. Also, cDNA from RNAs was synthesized by BioFact Kit (Daejeon, Korea).
Evaluation of the expression levels of mTOR mRNA, miR-101, and miR-214 was performed by quantitative real-time PCR method by using SYBR Green master mix (Ampliqon, Odense, Denmark) on LightCycler 96 system (Roche Company, Basel, Switzerland). Primers for target genes and GAPDH, as the reference gene, were designed by Primer Express 3.0 (Applied Biosystems, Foster City, CA, USA), (Table 1). The comparative CT method was employed to evaluate the relative amounts (using 2−∆∆CT formula) of target mRNA in the test samples, which were normalized to the corresponding GAPDH transcript level. Also, expression levels of miRNAs were normalized to the corresponding U6 as the housekeeping gene.
Table 1.
Primer sequences for studied genes
|
Primer
|
|
Sequence
|
| mTOR |
Forward |
5'-CTCAGGGCAAGATGCTTGGAA -3' |
| Reverse |
5'- CAGGACGCTCACATTGCTAGA-3' |
| GAPDH |
Forward |
5'- CAAGATCATCAGCAATGCCTCC-3' |
| Reverse |
5'-GCCATCACGCCACAGTTTCC -3' |
| miR-101 |
|
UACAGUACUGUGAUAACUGAA* |
| miR-214 |
|
ACAGCAGGCACAGACAGGCAGU* |
Statistical analysis
Obtained data were analyzed by using the GraphPad Prism software version 6.0.1 (GraphPad Prism, San Diego, CA, USA). After the calculation of the normality of data distribution using a one-way ANOVA test, the group comparisons were investigated through the Dunnett and Tukey nonparametric test. Data were represented as the mean ± standard deviation (SD) and P < 0.05 was considered the level of significance.
Results
Exosome characterization
Isolated Exosomes characteristics have been displayed in Figure 1 for morphology and specific protein markers. TEM results confirmed the morphology and size of the exosomes, and western blotting analysis showed their specific markers including CD9, CD81, and CD63 (Figure 1A, B, and C).
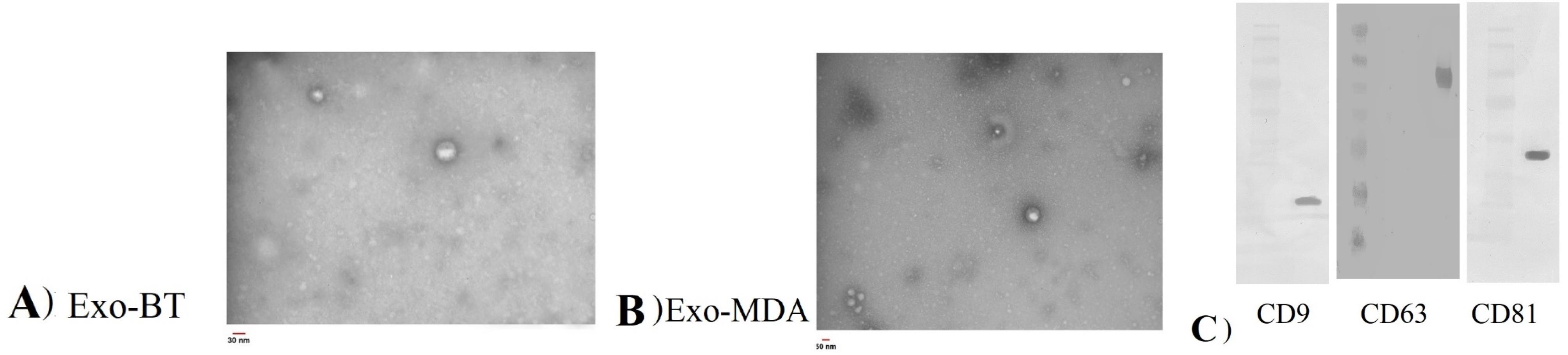
Figure 1.
Identification and characterization of exosomes derived from BT-474 (A and C) and MDA-MB-231 (B and C) cell lines
.
Identification and characterization of exosomes derived from BT-474 (A and C) and MDA-MB-231 (B and C) cell lines
Altered expression level of mTOR in BC cell lines after treatment with DHA
Expression of mTOR in BC cell lines and their derived exosomes were evaluated (Figure 2). The level expression of mTOR gene in the MDA-MB-231 cell line was much more than in BT-474. About exosomes derived from these two cell lines, this expression pattern was also true (Figure 2).
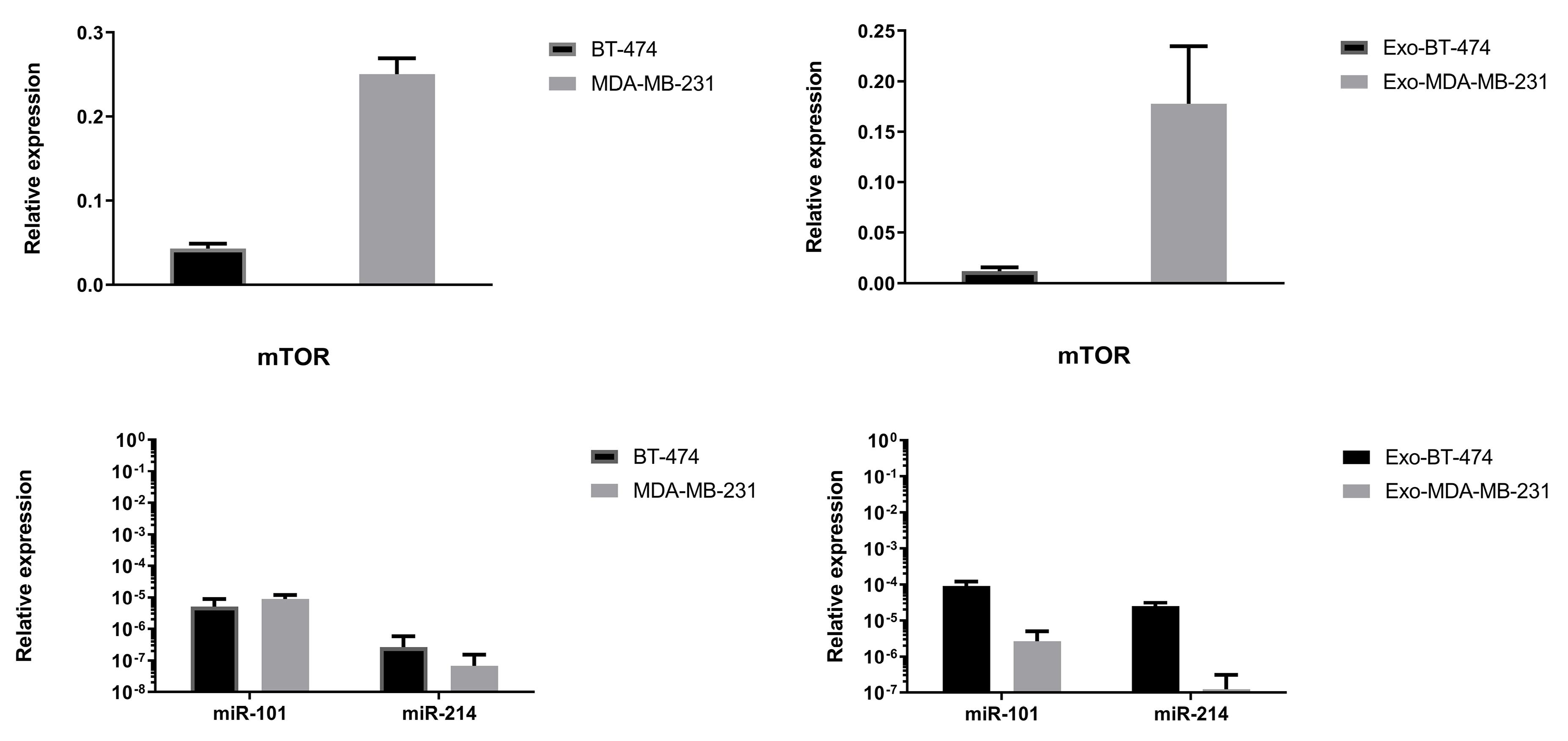
Figure 2.
Relative expression level of mTOR and related miRs in studied BC cell lines and exosomes derived from their (control groups)
.
Relative expression level of mTOR and related miRs in studied BC cell lines and exosomes derived from their (control groups)
Treatment with 100 μM DHA led to the significantly reduced expression of mTOR in BT-474 (-12787.72-fold, P= 0.0029) and MDA-MB-231 cells (-2.9686 fold, P < 0.0001) and exosomes derived from them (-2.3117 fold, P= 0.0002, -131578.94 fold, P < 0.0001, respectively) in normoxic condition (Figure 3, Table 2). Cellular and exosomal expression of mTOR was up-regulated under hypoxic conditions in both cell lines (Figure 3, Table 2). In this condition, DHA down-regulated the expression of mTOR in both cell lines and exosomes derived from them (Figure 3, Table 2).
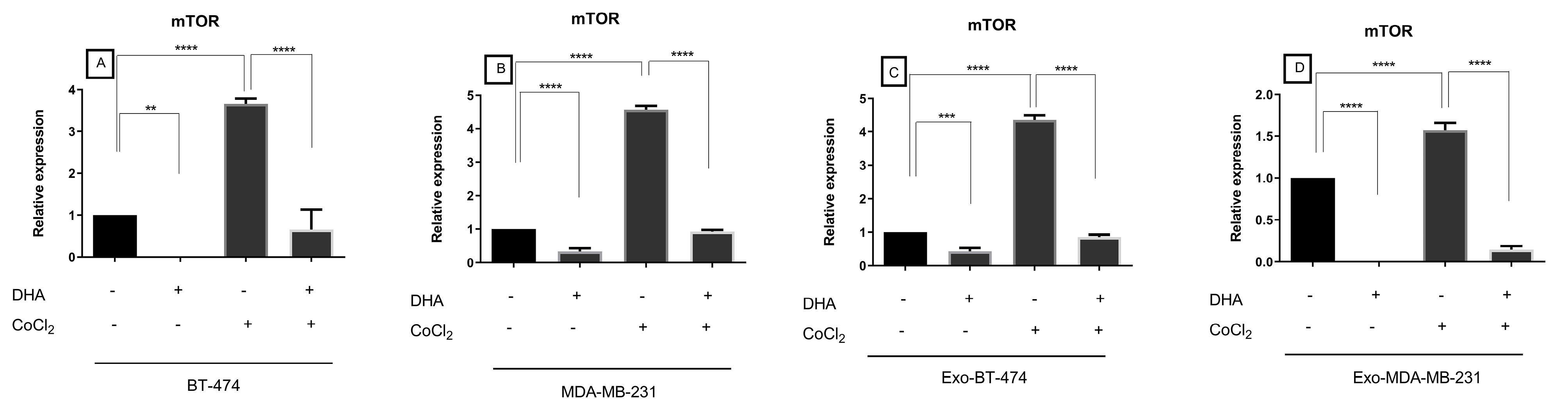
Figure 3.
Expression of mTOR in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
.
Expression of mTOR in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
Table 2.
Expression of mTOR in BC cell lines and exosomes derived from them after treatment with DHA under both normoxic and hypoxic conditions
|
mTOR
|
|
Normoxic
|
Hypoxic
|
|
Treated with DHA
|
Treated with CoCl2
|
Treated with DHA and CoCl2
|
|
|
Fold change
|
P
value*
|
Fold change
|
P
value**
|
Fold change
|
P
value***
|
| BT-474 |
-12787.72 |
0.0029 |
3.657109 |
< 0.0001 |
-5.5762 |
< 0.0001 |
| MDA-MB-231 |
-2.9686 |
< 0.0001 |
4.572474 |
< 0.0001 |
-4.9239 |
< 0.0001 |
| Exo-BT-474 |
-2.3117 |
0.0002 |
4.35161 |
< 0.0001 |
-5.0705 |
< 0.0001 |
| Exo-MDA-MB-231 |
-131578.94 |
< 0.0001 |
1.56912 |
< 0.0001 |
-10.7567 |
< 0.0001 |
* Compared control with DHA treated group.
** Compared control with CoCl2 treated group.
*** Compared CoCl2 treated group with DHA + CoCl2 treated group.
Altered expression level of miR-101 in BC cell lines after treatment with DHA
Level expression of miR-101 in control groups was shown more expression in MDA-MB-231 cell lines, and Exo-BT-474 (Figure 2). Expression of miR-101 as a regulator of mTOR, showed alteration consistent with the expression of mTOR i.e. down-regulation by hypoxia, and up-regulation by DHA (Figure 4, Table 3). Significantly, DHA increased expression of this tumor suppressor in BT-474, Exo-BT-474, and Exo-MDA-MB-231 (796.1783 fold, P < 0.0001, 1.660854 fold, P = 0.0237, 235.6823 fold, P < 0.0001, respectively). In hypoxic conditions, a reduction of expression of miR-101 was seen in MDA-MB-231, and Exo-BT-474 (-5.9145 fold, P < 0.0001, -15.3515 fold, P = 0.0035, respectively). Interestingly, treated with DHA and CoCl2 revealed up-regulation of miR-101 in BT-474 (11.9904 fold, P < 0.0001), and Exo-MDA-MB-231, (5.9312 fold, P = 0.0001). An unexpected result was the expression of miR-101 in MDA-MB-231 after treatment with DHA alone, and a combination of CoCl2 and DHA (Figure 4, Table 3).
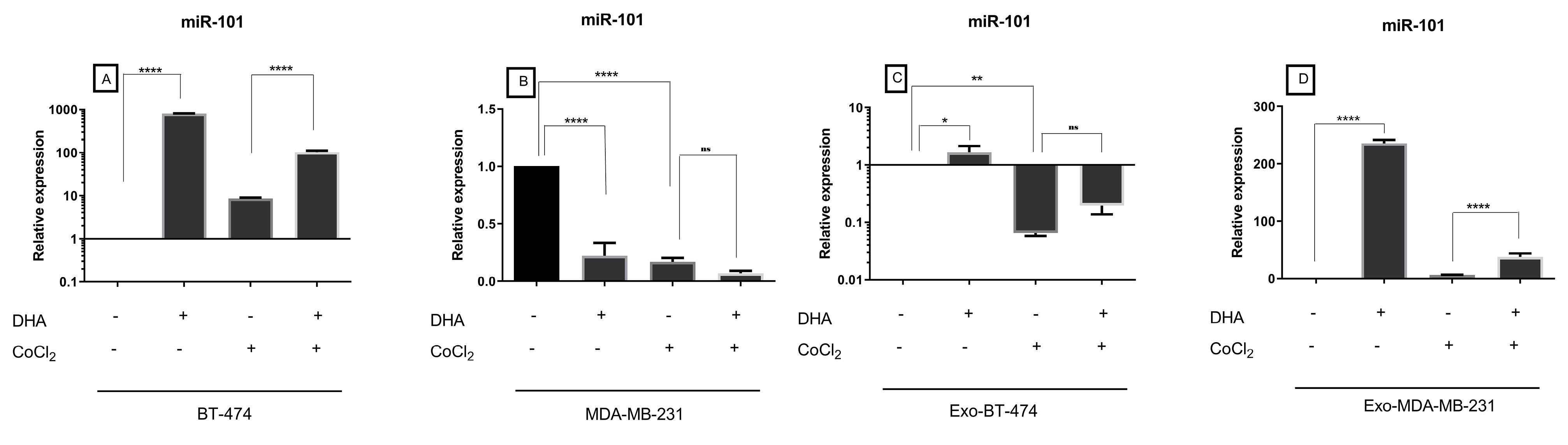
Figure 4.
Expression of miR-101 in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
.
Expression of miR-101 in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
Table 3.
Expression of miR-101 in BC cell lines and exosomes derived from them after treatment with DHA under both normoxic and hypoxic conditions
|
MiR-101
|
|
Normoxic
|
Hypoxic
|
|
Treated with DHA
|
Treated with CoCl2
|
Treated with DHA and CoCl2
|
|
|
Fold change
|
P
value*
|
Fold change
|
P
value**
|
Fold change
|
P
value***
|
| BT-474 |
796.1783 |
< 0.0001 |
8.6281 |
0.3609 |
11.9904 |
< 0.0001 |
| MDA-MB-231 |
-4.5020 |
< 0.0001 |
-5.9145 |
< 0.0001 |
-2.386 |
NS |
| Exo-BT-474 |
1.660854 |
0.0237 |
-15.3515 |
0.0035 |
3 |
NS |
| Exo-MDA-MB-231 |
235.6823 |
< 0.0001 |
6.3412 |
0.3611 |
5.9312 |
0.0001 |
* Compared control with DHA treated group.
** Compared control with CoCl2 treated group.
*** Compared CoCl2 treated group with DHA + CoCl2 treated group.
Altered expression level of miR-214 in BC cell lines after treatment with DHA
Another studied miRNA that regulates the expression of mTOR was miR-214. The expression of miR-214 in BT-474 was higher than the MDA-MB-231 (Figure 2). Also, there was a significantly higher expression of this tumor suppressor in the Exo-BT-474 than in the Exo-MDA-MB-231 (Figure 2). Importantly, its cellular and exosomal expression was also reduced after treatment with CoCl2 (Figure 5, Table 4). On the other hand, DHA led to the up-regulation of the expression of miR-214 in BC cell lines and their exosomes under both hypoxic and normoxic conditions (Figure 5, Table 4).
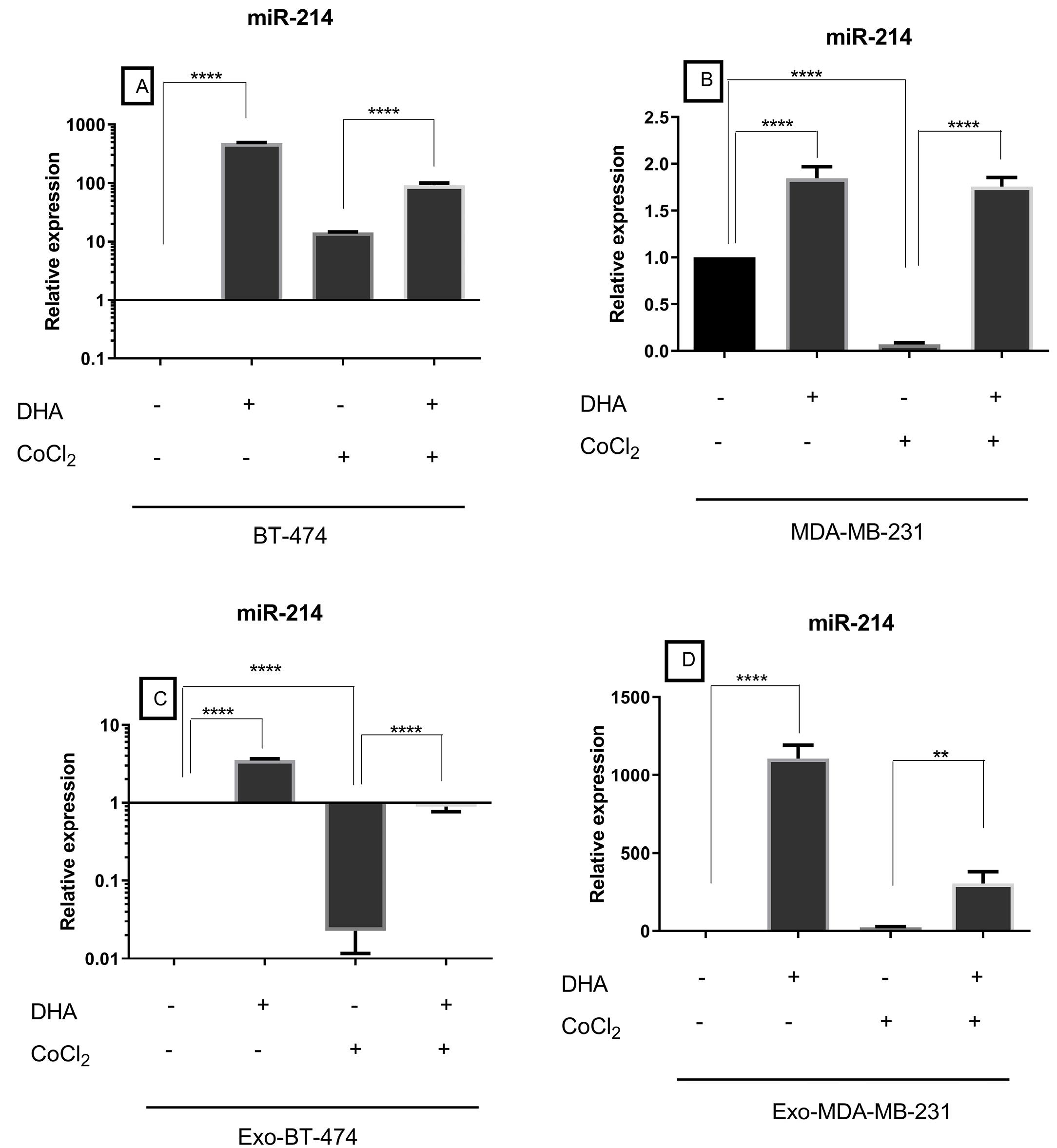
Figure 5.
Expression of miR-214 in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
.
Expression of miR-214 in treated BC cell lines and exosomes-derived from them after treatment with DHA under both normoxic and hypoxic conditions
Table 4.
Expression of miR-214 in BC cell lines and exosomes derived from them after treatment with DHA under both normoxic and hypoxic conditions
|
MiR-214
|
|
Normoxic
|
Hypoxic
|
|
Treated with DHA
|
Treated with CoCl2
|
Treated with DHA and CoCl2
|
|
|
Fold change
|
P
value*
|
Fold change
|
P
value**
|
Fold change
|
P
value***
|
| BT-474 |
484.0271 |
< 0.0001 |
14.3885 |
0.1016 |
6.4185 |
< 0.0001 |
| MDA-MB-231 |
1.8464 |
< 0.0001 |
-14.2957 |
< 0.0001 |
25.1889 |
< 0.0001 |
| Exo-BT-474 |
3.5436 |
< 0.0001 |
-44.016 |
< 0.0001 |
39.1543 |
< 0.0001 |
| Exo-MDA-MB-231 |
11064.6396 |
< 0.0001 |
23.0468 |
0.9334 |
13.1926 |
0.0014 |
* Compared control with DHA treated group.
** Compared control with CoCl2 treated group.
*** Compared CoCl2 treated group with DHA + CoCl2 treated group.
Discussion
Cancer exosomes have been gaining much attention due to have crucial impact on cancer progression. Our study showed that DHA significantly down-regulated mTOR expression in BC cell lines and exosomes secreted by them. Furthermore, we evaluated the effect of CoCl2, artificially induced hypoxia, in cellular and exosomal expression of mTOR before and after treatment with DHA. Our results demonstrated that mTOR expression strikingly increased under hypoxic conditions in cell lines and their exosomes and reduced expression level after treatment with DHA.
Recent evidence reveals that some genes such as mTOR pathway genes in exosomes derived from cancer cells can act as potential candidates for cancer therapy.13 Growth factor receptors including insulin receptors and human epidermal growth factor receptors activate PI3K, which activates Akt and subsequently mTOR pathway.14 This pathway has striking roles in cancer pathogenesis and irregularity in PI3K/AKT/mTOR signaling is prevalent in BC. So, the mTOR pathway has been recommended as a therapeutic target by some researchers.14 Signaling through PI3K/AKT/mTOR affects various cellular processes including survival, proliferation, metabolism, invasion, and chemoresistance has been proven to have important roles in the triggering and development of mammary tumorigenesis.14 Zhang et al have shown that there was significantly higher expression of PI3k, Akt, and mTOR genes in cervical cancer tissues and their secreted exosomes than in normal tissues.13 In another study, researchers loaded tumor suppressor miR (miR-7-5p, targets MAP kinase-interacting serine/threonine-protein kinase 1) in exosomes from non-small cell lung cancer (NSCLC).15 They showed that using a specific mTOR inhibitor (Everolimus) induced NSCLC to secrete miR-7-5p loaded exosome so, abrogation of the mTOR pathway was seen.15 Also, it has been reported that transferring of exosomal tumor suppressor miR (miR-100 and miR-143 decreased expression of mTOR, K-RAS, Cyclin D1, HK2, and increased expression of p-27) into colorectal tumor cells resulted in suppression of proliferation.16 A recent review of the literature on this subject found that n-3 PUFAs can play a negative role in the regulation of the PI3K/Akt pathway which, in turn, causes mTORC1 suppression.17 Tsai et al proved that DHA induced apoptosis through decreasing mTOR expression in MCF-7 cells.18 Also, Chénais et al demonstrated that DHA has been pro-apoptotic effect in MDA-MB-231 cells.19 In this report, the anti-proliferative mechanism of DHA is clarified by the downregulation of genes involved in the cholesterol biosynthesis pathway and the upregulation of endoplasmic reticulum-stress response.19 In a pilot study, researchers showed that consuming n-3 LCPUFAs 3.4 g/d for 6 months resulted in a significantly decreased protein involved in BC such as AKT/mTOR pathway.20 In another study, treatment of glioblastoma cell lines with DHA showed that 5’-AMP-activated protein kinase (AMPK) was activated and levels of phosphorylated Akt and mTOR were decreased 21. In this study, in vivo research proved that expression of Caenorhabditis elegans ω3-desaturase, an enzyme that converts n-6 PUFAs to n-3 PUFAs, was strikingly reduced in fat-1 transgenic mice compared with wild-type.21 Also, TUNEL-positive cell numbers were increased in fat-1 transgenic mice in tumor sites correlated to wild-type.21 Shin et al reported that DHA reduced the levels of phospho-Akt and phospho-mTOR in a concentration-dependent manner in prostate cancer cells, hence inducing autophagy-associated apoptotic cell death.22 Kim et al indicated that DHA-induced apoptosis and autophagy in NSCLC cells were associated with mTOR suppression.23 Tamarindo andGóes reported that DHA has an anti-proliferative effect through metabolism modulation and androgen-regulated genes in prostate cancer.24 Moreover, several studies reported other anti-cancer effects of DHA, in BC in vivo and in vitro experiments.25,26 Aslan et al proved that DHA has an anti-angiogenesis effect in cellular and exosomal levels in BC cell lines.27 Research in the treatment of TNBC with DHA demonstrated a blocking effect on the VEGFR-2/PI3K/Akt/mTOR signaling pathway, subsequently downregulation the level of NF-κB and mutant p53, and elevated autophagy in this type of BC. Also, induction of cell cycle suppression has been observed through p21-mediated block of CDK1,2 activity.28 In addition, DHA caused an increase of PTEN in mRNA and protein level of expression so the phosphorylation of the p65 subunit of NF-kB was restrained and VEGF expression was reduced in MDA MB-231 cells.28 Anti-immunosuppressive effect of DHA has been reported by Fadaee et al.29 In his analysis, DHA suppressed the level expression of immune checkpoint molecules in both normoxic and hypoxic conditions in colorectal cancer cells.29 Furthermore, lipid deregulation, cell cycle impairment, and oxidative stress were other effects of DHA.24
The inverse correlation between mTOR expression and miR-101 and miR-214 expression has been validated in several types of human carcinomas.30-32 In a study done by Gu et al miR-101-3p regulated cullin-4B within PI3K/AKT/mTOR signaling pathway on development of prostate cancer.33 In another study, increasing expression of ataxia telangiectasia mutated (ATM) and mTOR by the negative regulation of miR-101 expression has been validated.30 In NSCLC, mTOR, p-S6, and p-mTOR expression are repressed via overexpression of miR-101-3p.34 About miR-214, p-Akt, and p-mTOR in gastric cancer cells were inhibited via mimics of this miR.35 Treated colon cancer cells with miR-214 mimic molecules proved that the downregulation of several oncogenic markers such as mTOR, cyclin D1, β-catenin, and c-Myc.36 However, treated colon cancer cells with miR-214 inhibitor showed reverse results.36
Collectively, our study indicated DHA is able to alteration of BC cells and their exosome contents under both normoxic and hypoxic conditions. This data suggests the anti-progressive effect of DHA in BC through decreasing expression of exosomal mTOR, and increasing miR-214.
Conclusion
In the current study, the expression level of mTOR and its targeting miRNAs was evaluated in BC cells and purified exosomes after DHA treatment. It was observed that DHA resulted in the downregulation of mTOR expression both in the BC cell lines as well as related exosomes, while the expression of mTOR targeting miRNAs was increased. DHA increased miR-101 and miR-214 other than Exo-BT in hypoxic conditions. Further, miR-101 did not increase in MDA-MB-231. These miRNAs might be involved in the regulation of mTOR which could be the underlying effect of DHA therapy.
Acknowledgements
The authors wish to thank the financial support from the Immunology Research Center, Tabriz University of Medical Science, Tabriz, Iran.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
Not applicable.
References
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019; 321(3):288-300. doi: 10.1001/jama.2018.19323 [Crossref] [ Google Scholar]
- Wang TT, Yang Y, Wang F, Yang WG, Zhang JJ, Zou ZQ. Docosahexaenoic acid monoglyceride induces apoptosis and autophagy in breast cancer cells via lipid peroxidation-mediated endoplasmic reticulum stress. J Food Sci 2021; 86(10):4704-16. doi: 10.1111/1750-3841.15900 [Crossref] [ Google Scholar]
- Anjom-Shoae J, Sadeghi O, Larijani B, Esmaillzadeh A. Dietary intake and serum levels of trans fatty acids and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Clin Nutr 2020; 39(3):755-64. doi: 10.1016/j.clnu.2019.03.024 [Crossref] [ Google Scholar]
- Ashfaq W, Rehman K, Siddique MI, Khan Q-A-A. Eicosapentaenoic acid and docosahexaenoic acid from fish oil and their role in cancer research. Food Rev Int 2020; 36(8):795-814. doi: 10.1080/87559129.2019.1686761 [Crossref] [ Google Scholar]
- Chen KM, Thompson H, Vanden-Heuvel JP, Sun YW, Trushin N, Aliaga C. Lipoxygenase catalyzed metabolites derived from docosahexaenoic acid are promising antitumor agents against breast cancer. Sci Rep 2021; 11(1):410. doi: 10.1038/s41598-020-79716-x [Crossref] [ Google Scholar]
- Andrade-Vieira R, Han JH, Marignani PA. Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1. Cancer Biol Ther 2013; 14(11):1050-8. doi: 10.4161/cbt.26206 [Crossref] [ Google Scholar]
- Taherian-Esfahani Z, Taheri M, Dashti S, Kholghi-Oskooei V, Geranpayeh L, Ghafouri-Fard S. Assessment of the expression pattern of mTOR-associated lncRNAs and their genomic variants in the patients with breast cancer. J Cell Physiol 2019; 234(12):22044-56. doi: 10.1002/jcp.28767 [Crossref] [ Google Scholar]
- Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci 2019; 20(3):755. doi: 10.3390/ijms20030755 [Crossref] [ Google Scholar]
- Ortega MA, Fraile-Martínez O, Asúnsolo Á, Buján J, García-Honduvilla N, Coca S. Signal transduction pathways in breast cancer: the important role of PI3K/Akt/mTOR. J Oncol 2020; 2020:9258396. doi: 10.1155/2020/9258396 [Crossref] [ Google Scholar]
- Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T. Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol 2019; 234(10):16885-903. doi: 10.1002/jcp.28374 [Crossref] [ Google Scholar]
- Pan S, Zhao X, Shao C, Fu B, Huang Y, Zhang N. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis 2021; 12(1):38. doi: 10.1038/s41419-020-03304-0 [Crossref] [ Google Scholar]
- Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012(59):e3037. 10.3791/3037.
- Zhang W, Zhou Q, Wei Y, Da M, Zhang C, Zhong J. The exosome-mediated PI3K/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol 2019; 12(7):2474-84. [ Google Scholar]
- Donovan MG, Selmin OI, Stillwater BJ, Neumayer LA, Romagnolo DF. Do olive and fish oils of the mediterranean diet have a role in triple negative breast cancer prevention and therapy? An exploration of evidence in cells and animal models. Front Nutr 2020; 7:571455. doi: 10.3389/fnut.2020.571455 [Crossref] [ Google Scholar]
- Liu S, Wang W, Ning Y, Zheng H, Zhan Y, Wang H. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death Dis 2022; 13(2):129. doi: 10.1038/s41419-022-04565-7 [Crossref] [ Google Scholar]
- Jahangiri B, Khalaj-Kondori M, Asadollahi E, Purrafee Dizaj L, Sadeghizadeh M. MSC-derived exosomes suppress colorectal cancer cell proliferation and metastasis via miR-100/mTOR/miR-143 pathway. Int J Pharm 2022; 627:122214. doi: 10.1016/j.ijpharm.2022.122214 [Crossref] [ Google Scholar]
- Shirooie S, Nabavi SF, Dehpour AR, Belwal T, Habtemariam S, Argüelles S. Targeting mTORs by omega-3 fatty acids: a possible novel therapeutic strategy for neurodegeneration?. Pharmacol Res 2018; 135:37-48. doi: 10.1016/j.phrs.2018.07.004 [Crossref] [ Google Scholar]
- Tsai CH, Lii CK, Wang TS, Liu KL, Chen HW, Huang CS. Docosahexaenoic acid promotes the formation of autophagosomes in MCF-7 breast cancer cells through oxidative stress-induced growth inhibitor 1 mediated activation of AMPK/mTOR pathway. Food Chem Toxicol 2021; 154:112318. doi: 10.1016/j.fct.2021.112318 [Crossref] [ Google Scholar]
- Chénais B, Cornec M, Dumont S, Marchand J, Blanckaert V. Transcriptomic response of breast cancer cells MDA-MB-231 to docosahexaenoic acid: downregulation of lipid and cholesterol metabolism genes and upregulation of genes of the pro-apoptotic ER-stress pathway. Int J Environ Res Public Health 2020; 17(10):3746. doi: 10.3390/ijerph17103746 [Crossref] [ Google Scholar]
- Fabian CJ, Kimler BF, Phillips TA, Box JA, Kreutzjans AL, Carlson SE. Modulation of breast cancer risk biomarkers by high-dose omega-3 fatty acids: phase II pilot study in premenopausal women. Cancer Prev Res (Phila) 2015; 8(10):912-21. doi: 10.1158/1940-6207.capr-14-0335 [Crossref] [ Google Scholar]
- Kim S, Jing K, Shin S, Jeong S, Han SH, Oh H. ω3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: in vitro and in vivo. Oncol Rep 2018; 39(1):239-46. doi: 10.3892/or.2017.6101 [Crossref] [ Google Scholar]
- Shin S, Jing K, Jeong S, Kim N, Song KS, Heo JY. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed Res Int 2013; 2013:568671. doi: 10.1155/2013/568671 [Crossref] [ Google Scholar]
- Kim N, Jeong S, Jing K, Shin S, Kim S, Heo JY. Docosahexaenoic acid induces cell death in human non-small cell lung cancer cells by repressing mTOR via AMPK activation and PI3K/Akt inhibition. Biomed Res Int 2015; 2015:239764. doi: 10.1155/2015/239764 [Crossref] [ Google Scholar]
- Tamarindo GH, Góes RM. Docosahexaenoic acid differentially modulates the cell cycle and metabolism- related genes in tumor and pre-malignant prostate cells. Biochim Biophys Acta Mol Cell Biol Lipids 2020; 1865(10):158766. doi: 10.1016/j.bbalip.2020.158766 [Crossref] [ Google Scholar]
- Newell M, Brun M, Field CJ. Treatment with DHA modifies the response of MDA-MB-231 breast cancer cells and tumors from nu/nu mice to doxorubicin through apoptosis and cell cycle arrest. J Nutr 2019; 149(1):46-56. doi: 10.1093/jn/nxy224 [Crossref] [ Google Scholar]
- Javadian M, Shekari N, Soltani-Zangbar MS, Mohammadi A, Mansoori B, Maralbashi S. Docosahexaenoic acid suppresses migration of triple-negative breast cancer cell through targeting metastasis-related genes and microRNA under normoxic and hypoxic conditions. J Cell Biochem 2020; 121(3):2416-27. doi: 10.1002/jcb.29464 [Crossref] [ Google Scholar]
- Aslan C, Maralbashi S, Kahroba H, Asadi M, Soltani-Zangbar MS, Javadian M. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci 2020; 258:118094. doi: 10.1016/j.lfs.2020.118094 [Crossref] [ Google Scholar]
- Ma Y, Wang J, Li Q, Cao B. The effect of omega-3 polyunsaturated fatty acid supplementations on anti-tumor drugs in triple negative breast cancer. Nutr Cancer 2021; 73(2):196-205. doi: 10.1080/01635581.2020.1743873 [Crossref] [ Google Scholar]
- Fadaee M, Abbasi H, Maralbashi S, Baradaran B, Shanehbandi D, Faghih Dinevari M. Docosahexaenoic acid may inhibit immune evasion of colorectal cancer cells through targeting immune checkpoint and immunomodulator genes and their controlling microRNAs. Biofactors 2022; 48(5):1137-44. doi: 10.1002/biof.1842 [Crossref] [ Google Scholar]
- Chen M, Liu P, Chen Y, Chen Z, Shen M, Liu X. Long noncoding RNA FAM201A mediates the radiosensitivity of esophageal squamous cell cancer by regulating ATM and mTOR expression via miR-101. Front Genet 2018; 9:611. doi: 10.3389/fgene.2018.00611 [Crossref] [ Google Scholar]
- Zhang S, Wang M, Li Q, Zhu P. miR-101 reduces cell proliferation and invasion and enhances apoptosis in endometrial cancer via regulating PI3K/Akt/mTOR. Cancer Biomark 2017; 21(1):179-86. doi: 10.3233/cbm-170620 [Crossref] [ Google Scholar]
- Wang F, Tan WH, Liu W, Jin YX, Dong DD, Zhao XJ. Effects of miR-214 on cervical cancer cell proliferation, apoptosis and invasion via modulating PI3K/AKT/mTOR signal pathway. Eur Rev Med Pharmacol Sci 2020; 24(14):7573. doi: 10.26355/eurrev_202007_22242 [Crossref] [ Google Scholar]
- Gu Z, You Z, Yang Y, Ding R, Wang M, Pu J. Inhibition of microRNA miR-101-3p on prostate cancer progression by regulating Cullin 4B (CUL4B) and PI3K/AKT/mTOR signaling pathways. Bioengineered 2021; 12(1):4719-35. doi: 10.1080/21655979.2021.1949513 [Crossref] [ Google Scholar]
- Li Z, Qu Z, Wang Y, Qin M, Zhang H. miR-101-3p sensitizes non-small cell lung cancer cells to irradiation. Open Med (Wars) 2020; 15(1):413-23. doi: 10.1515/med-2020-0044 [Crossref] [ Google Scholar]
- Tao W, Li Y, Zhu M, Li C, Li P. LncRNA NORAD promotes proliferation and inhibits apoptosis of gastric cancer by regulating miR-214/Akt/mTOR axis. Onco Targets Ther 2019; 12:8841-51. doi: 10.2147/ott.s216862 [Crossref] [ Google Scholar]
- Wu SY, Huang YJ, Tzeng YM, Huang CF, Hsiao M, Wu ATH. Destruxin B suppresses drug-resistant colon tumorigenesis and stemness is associated with the upregulation of miR-214 and downregulation of mTOR/β-catenin pathway. Cancers (Basel) 2018; 10(10):353. doi: 10.3390/cancers10100353 [Crossref] [ Google Scholar]