ImmunoAnalysis. 4:7.
doi: 10.34172/ia.4080
Original Article
Comparative Study of the Effects of Docosahexaenoic Acid, Linoleic Acid, and Taxol in Decreasing the Expression of miR-10b, and miR-20a OncomiRs, and their Target Major Histocompatibility Complex Class I Chain-Related Proteins A (MICA) in Triple-Negative Metastatic Breast Cancer Cell Line
Sepideh Maralbashi Investigation, Methodology, Writing – original draft, 1, 2 
Farhad Salari Funding acquisition, Validation, Visualization, 1
Cynthia Aslan Investigation, Methodology, Writing – review & editing, 3
Najibeh Shekari Investigation, Methodology, Writing – review & editing, 3
Mahsa Javadian Data curation, Methodology, Writing – review & editing, 4
Milad Asadi Formal analysis, Software, 3
Tohid Kazemi Conceptualization, Supervision, Validation, 3, * 
Author information:
1Department of Immunology, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
3Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Immunology, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Background:
Molecular signatures of the effects of widely used omega-3 and omega-6 fatty acids as dietary supplements and even in cancer therapy have been studied. Of them, microRNAs, have been shown to undergo altered expression after treatment with docosahexaenoic acid (DHA) and linoleic acid (LA) in different cancer types. In this study, the expression level of two well-known onco-microRNAs (miR), miR-10b, miR-20a, and also major histocompatibility complex class I chain-related protein A (MICA) as the target for these oncomiRs were investigated after treatment with DHA, LA, and common therapeutic agent Taxol .
Methods:
MDA-MB-231 cells were cultured in a complete RPMI 1640 medium and an MTT assay was performed to determine IC50 of DHA, LA, and Taxol. Cells were treated by DHA (100µM), LA (50µM), and Taxol (0.3µM) alone or in different combinations. After RNA extraction and cDNA synthesis, the expression levels of miR-10b, miR-20a, and MICA were determined by quantitative real-time PCR. U6 and β-Actin were used as endogenous controls, respectively.
Results:
Our findings showed that DHA, LA, and Taxol, and their combinations led to the significantly decreased expression of miR-10b and miR-20a (all P<0.0001). The expression level of miR-10b and miR-20a significantly decreased when treated with the LA+TAX combination. MICA as the target of miR-10b and miR-20a was also down-regulated significantly (P<0.0001) especially when treated with DHA+LA+TAX.
Conclusion:
Useful effects of DHA, LA, and their combination in breast cancer (BC) could be interpreted in part by decreasing the expression level of oncomiRs. In the case of MICA have had some contrary. High expression of MICA/B caused an approving outcome concerning the relapse-free period in early BC patients, however; some studies have shown that higher MICA expression was found as a sign of poor prognosis in BC. The decreased expression of MICA in the present study suggests the potential role of DHA and LA should be used in dietary supplements of BC patients.
Keywords: Breast cancer, Docosahexaenoic acid, Linoleic acid, miR-10b, miR-20a, MICA
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was performed by grants from Kermanshah University of Medical Sciences (grant number: 97920).
Introduction
In women, breast cancer (BC) accounts for the highest diagnosis number of patients in worldwide. Annually, 1.68 million new cases are diagnosed and 522 000 patients pass away.1 This heterogeneous disease has been classified four molecular subtypes base on expression human epidermal growth factor receptor 2 (HER2), progesterone receptor (PR), and estrogen receptor (ER) 2. Generally, triple-negative BC (TNBC) accounts for 15%–20% of all BC. In spite of optimum local and systemic treatments, TNBC are recognized to be an aggressive class with upper rates of relapse compared with HER2- and ER/PR-positive BC.3-6
Most epidemiological studies have recommended that a healthy lifestyle and dietary fatty acids are crucial for diminishing the risk of BC.7-9 There are two main classes of polyunsaturated fatty acids (PUFAs): n-3 PUFA and n-6 PUFA.9,10 In mammalian, n-3 and n-6 PUFAs are necessary fatty acids and because of they cannot synthesized endogenously, must be consumed in the diet.11 Linoleic acid (LA) (18:2 n-6) is the well-known n-6 PUFA.12,13 Recent studies zoom in effects of LA on biology of cancer cells demonstrated that LA repressed BC proliferation in both in vitro and in vitro; however, creating the results about inferring on a carcinogenic part for LA in pathology of BC has caused its protective role to be questionable 14. N-3 PUFAs including docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) can be gained from marine foods such as fish oil and seafood.13 DHA has been detailed to have advantageous impacts for treating different sorts of cancers along with the approved treatment regimens.14 Beneficial effects of DHA such as reduction of inflammation, suppression of tumor cell proliferation, and induction of apoptosis have been reported. DHA regulates immune responses in favor of decreasing inflammation through inhibition of pro-inflammatory mediatorsandinduction of adiponectin.14 Also, DHA has been appeared to repress the invasion of the MDA‐MB‐231 cells via increasing of expression of the cytokeratin‐1. In addition, DHA causes activation of peroxisome proliferator‐activated receptors α (PPAR‐α)/TLR‐4 pathways so apoptosis occurs in MDA‐MB‐231 cells and down-regulation expression of miR-20a, one of the important miRs in progression and metastasis of BC.14
Repression of gene expression usually do by a sort of short non-coding RNA molecules called miRs. These molecules bind to the 3’-untranslated region (3’-UTR) of target mRNAs. Evidences provide that miRNAs have significant functions in formation and immunogenicity of tumor.15 Recent findings regarding miR-20a, miR-106b, and miR-93 have led to suppressing MICA, and MICB expression and contributed to immune evasion.16,17 The inappropriate expression of some miRs such as miR-20b, miR-138, miR-190b and miR-29 has been observed in breast carcinoma, particularly in TNBC. The miR-20a, consists of miRNA-20a-3p and miRNA-20a-5p, which belongs to the miR-17-92 gene cluster. MiR-20a is conserved in the human genome and plays substantial role in tumor genesis and its development. It has been inferred that the expression of miR-20a-5p is upregulated in hepatocellular carcinoma and gastric diseases, however, the decreased expression of miR-20a-5p is indicated in breast carcinoma.18 miR-20a downregulated ULBP2 and the expression of MICA/B by inhibiting the MAPK/ERK signaling pathway and targeting the MICA/B 3’-untranslated region, respectively. Functional analysis indicates that the silencing of NKG2DL-targeting miRNAs in BC cells increases inhibited immune escape in vivo and NK cell-mediated cytotoxicity in vitro. moreover, histone deacetylase inhibitors (HDACis) increase expression of NKG2DL in BC cells because they inhibit the miR-17-92 cluster’s members. The miR-20a expression induces an abnormal vascular mesh formation and also angiogenesis in BC.14
miR-10b is another microRNA involved in this network which is remarkably upregulated in human BC cells. It targets KLF4 and HOXD10 to play a pro-oncogenic role and can raise cell invasion and also metastasis formation in the TNBC subtype by its secretion via exosomal vesicles, mediated through neutral sphingomyelin phosphodiesterase 2 (nSMase) and also it can transform non-malignant HMLE cells into the type of cells with invasion-ability. Directly inhibition of miRNA target, PTEN, and on the other hand the indirectly enhance of the expression of AKT, as well as that of BCL2 like 11(BIM) and HOXD10 causes Metastasis generation and self-renewal of cancer stem-like cells driven by miR-10b.19
miR-10b is an interesting candidate due to its close correlation with the metastatic behaviors. The subsequent researches on miR-10b validate its candidacy as a mechanistically significant miRNA, as demonstrated through in vivo experiments indicating that miR-10b overexpression in otherwise non-metastatic breast tumors trigger tumor invasion and also distant metastasis in the xenotransplantation models. Conversely, metastasis is suppressed in a model of mouse mammary tumor by therapeutic silencing of miR-10b.20 Therefore, targeting miRNAs with the antisense inhibitors represents a new approach to enhance the immunogenicity of BC.21
Because of insufficient knowledge in the field of the molecular targets related to the anticancer effect of DHA, this study is designed to study the DHA effect (alone and combined with the chemotherapeutic agent, Taxol) on the expression level of the immune‐related gene, MICA. miR‐10b and miR‐20a, as two miRs are also involved in this study, due to their roles in BC development and their relations with the MICA expression.
In the present study, we found that the DHA, LA, and Taxol (generic name paclitaxel), and their combinations significantly down-regulated expression of miR-10b and miR-20a in MDA-MB-231 cells. The level expression of MICA also decreased.
Methods and Materials
Cell culture and reagents
Human highly metastatic BC cell line, MDA-MB-231 was purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). Cell line was cultured in complete Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco Inc., El Paso, TX) supplemented by 10% of fetal bovine serum (Gibco Inc.), 100 unit/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 37 °C in a 95% humidified incubator with 5% CO2. Cells with 80% confluency were treated with 100 µM DHA (Sigma-Aldrich) and/or 50µM LA (Sigma-Aldrich) and/or 0.3µM Taxol for 24 hours. Cells treated with vehicle buffer were considered as control cells.
MTT cell proliferation assay
MDA-MB-231 cell line was seeded at a density of 15 × 103 cell/well in 200 μL culture medium at 96-well tissue culture plates. The next day medium was replaced with 200 μL of fresh media comprising different concentration of Taxol (0.1 nM, 1 nM, 10 nM, 100 nM, 1 μM, 10 μM and 100 μM) and incubated for 24 hours at 37 °C and 5% CO2. Following 24 hours the medium was removed and the cells were washed with phosphate-buffered saline (PBS) and 50 μL of 2 mg/mL MTT solution (Sigma–Aldrich, St. Louis, MO) with 150 μL media was added to every well and incubated for 4 hours at 37 °C with 5% CO2. The solution was removed and dimethyl sulfoxide (DMSO) and Sorenson were added. Afterwards 30 minutes the plates were read at 570 nm in a microplate reader and Taxol IC50 (half maximal inhibitory concentration) for the MDA-MB-231 cell line was determined. To obtain a more accurate dose of Taxol in the following cell line, we limited the range of Taxol concentrations in the second step.
RNA extraction and cDNA synthesis
Cells were used for RNA extraction by the TRIzol method (RiboEX, GeneAll Biotechnology, Korea) recommended by the manufacturer. RNA concentration was evaluated by a Nano-Drop Spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA) and then kept at -80 °C for future experiments. Extracted RNA samples were applied to assess the extraction qualitatively by electrophoresis on an agarose gel to observe 18s and 28s ribosomal RNA bands. miRNAs were reverse transcribed by Universal cDNA Synthesis Kit II (Exiqon, Vedbaek, Denmark) based on the manufacturer’s instruction. Also, cDNA from RNAs was synthesized by BioFact Kit (Daejeon, Korea).
Quantitative real-time PCR
Evaluation of the expression levels of MICA mRNA, miR-10b, and miR-20a was performed by Quantitative Real-time PCR method by using the SYBR Green master mix (Yekta Tajhiz Azma, Tehran, Iran) on Light Cycler 96 system (Roche Company, Basel, Switzerland). MICA and miRNA primers were purchased from Bioneer (South Korea), and Exiqon respectively (Table 1). The comparative CT method was employed to evaluate the relative amounts (using the 2−∆∆CT formula) of target mRNA in the test samples, which were normalized to the corresponding β-actin transcript level. Also, Expression levels of miRNAs were normalized to the corresponding U6 as the housekeeping gene.
Table 1.
Primer sequences
|
Primer
|
Sequences
|
| MICA |
Forward |
5´- GACAGCTGACTTGCTGAGAGG-3' |
| Reverse |
5'- GGTAAACAGGAGCACGAGGAT-3' |
| β-Actin |
Forward |
5'-TCCCTGGAGAAGAGCTACG-3' |
| Reverse |
5'-GTAGTTTCGTGGATGCCACA-3' |
| U6* |
5'-GGG CAG GAA GAG GGC CTA T-3' |
| miR-10b* |
UACCCUGUAGAACCGAAUUUGUG |
| miR-20a* |
UAAAGUGCUUAUAGUGCAGGUAG |
Statistical analysis
Obtained data were analyzed by using the GraphPad Prism software version 6.0.1 (GraphPad Prism, San Diego, CA, USA). After the calculation of the normality of data distribution using unpaired student’s t test. The data were represented as the mean ± standard deviation (SD) and P < 0.05 was considered the level of significance.
Results
DHA, LA, and Taxol reduced highly metastatic BC cell line viability
To evaluate IC50 of DHA, LA, and Taxol in the MDA-MB-231 cell line, an MTT assay was used. The IC50 of Taxol, DHA, LA was 0.3 µM, 100 µM, and 50 µM respectively (Figure 1).
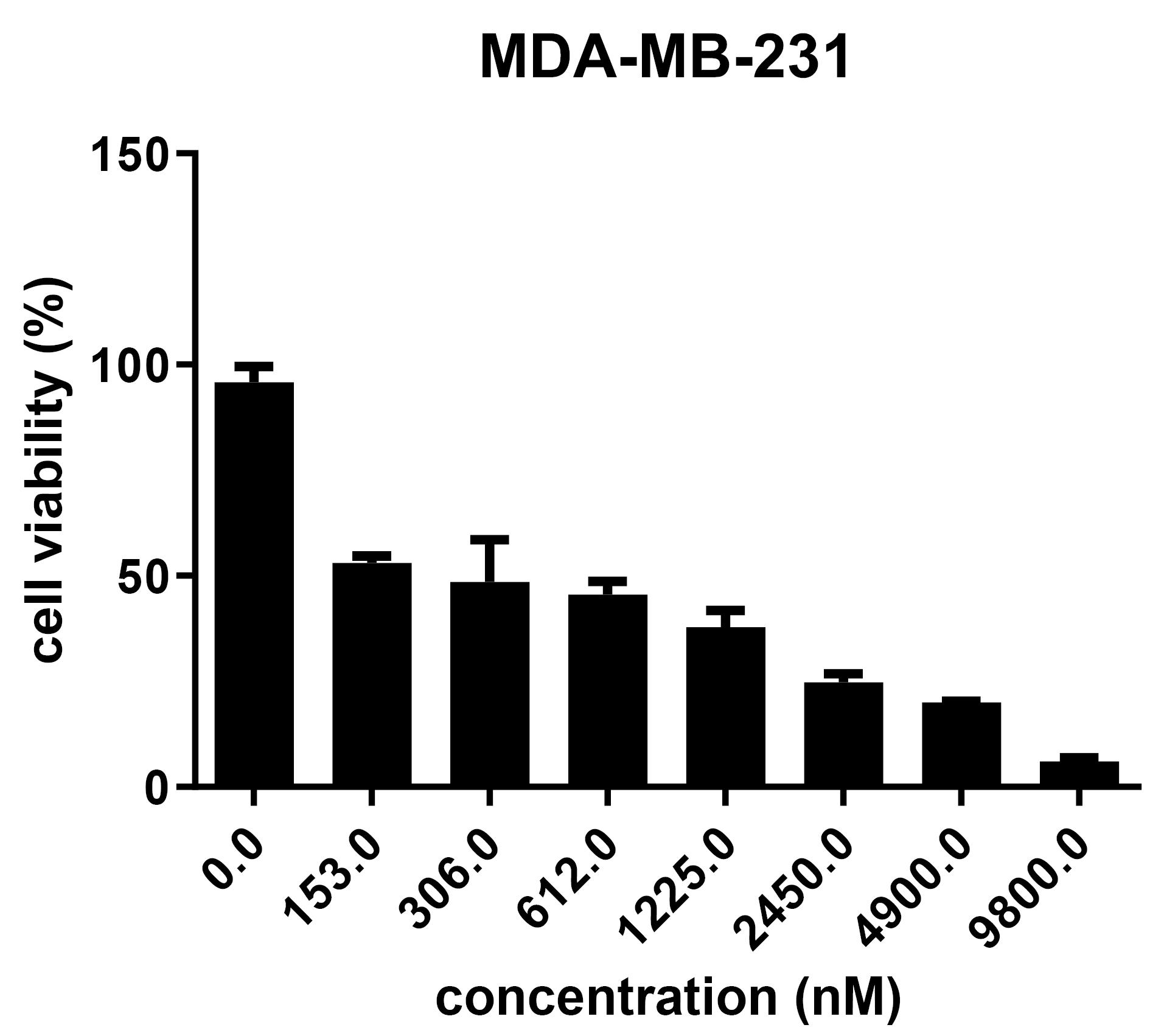
Figure 1.
Cytotoxicity effect of Taxol on MDA-MB-231 cells
.
Cytotoxicity effect of Taxol on MDA-MB-231 cells
DHA, LA, Taxol, and their combinations down-regulated the level expression of the oncomiRs in the MDA-MB-231 cell line
To determine which one of the drugs has the most impact on the expression of oncomiRs, we treated MDA-MB-231 cell lines with the mentioned drugs alone and their combinations. As shown in Figures 2 and 3 combinations of LA and Taxol significantly diminished the expression of miR-10b, and miR-20a (all P < 0.0001). Overall, these results indicate that the anti-cancer effect of DHA and LA strikingly increased when combined with Taxol (Table 2).
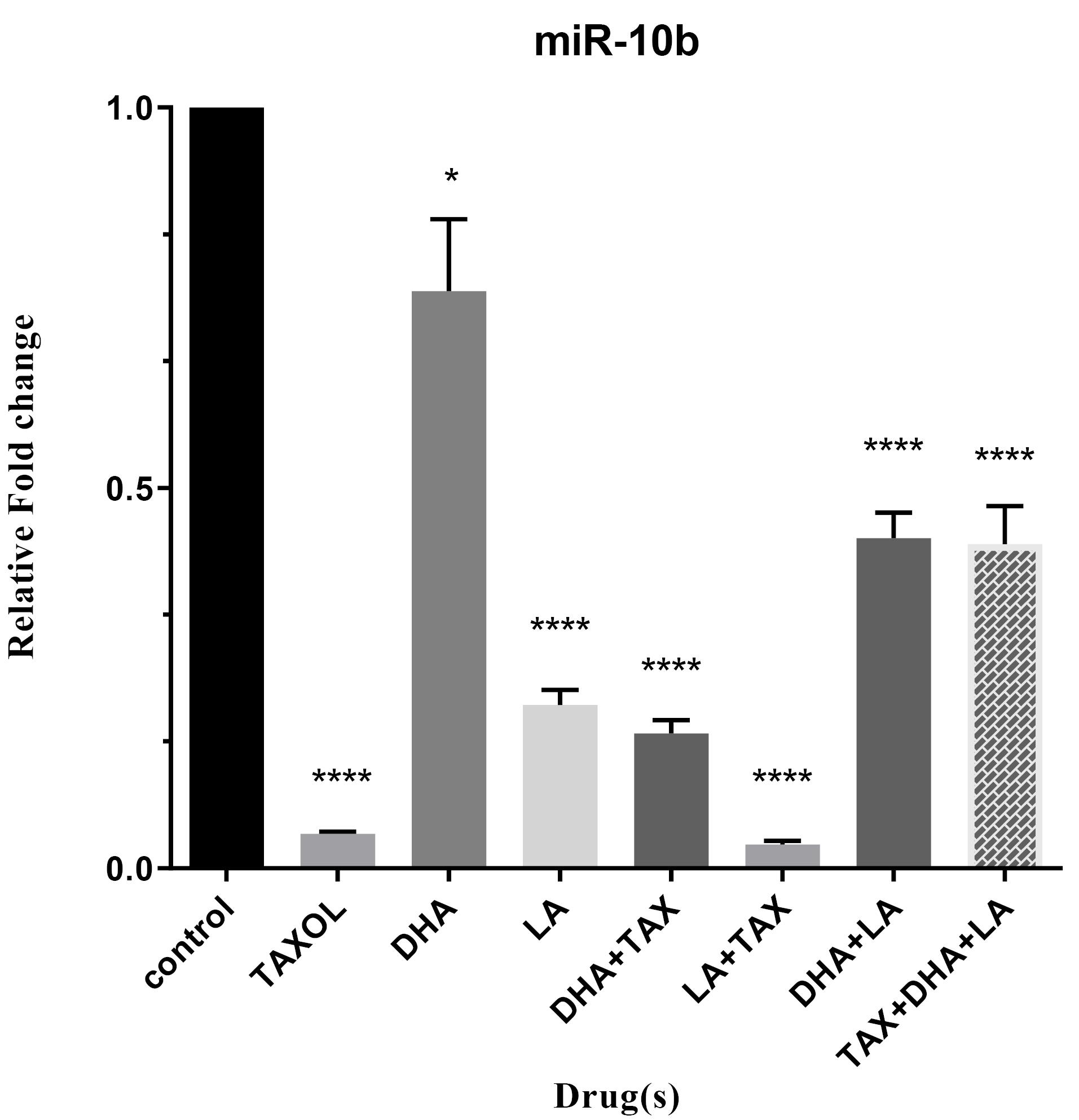
Figure 2.
Expression level of miR-10b in triple-negative metastatic human breast cancer cell line, MDA-MB-231, treated with DHA, LA, Taxol and their different combinations
.
Expression level of miR-10b in triple-negative metastatic human breast cancer cell line, MDA-MB-231, treated with DHA, LA, Taxol and their different combinations
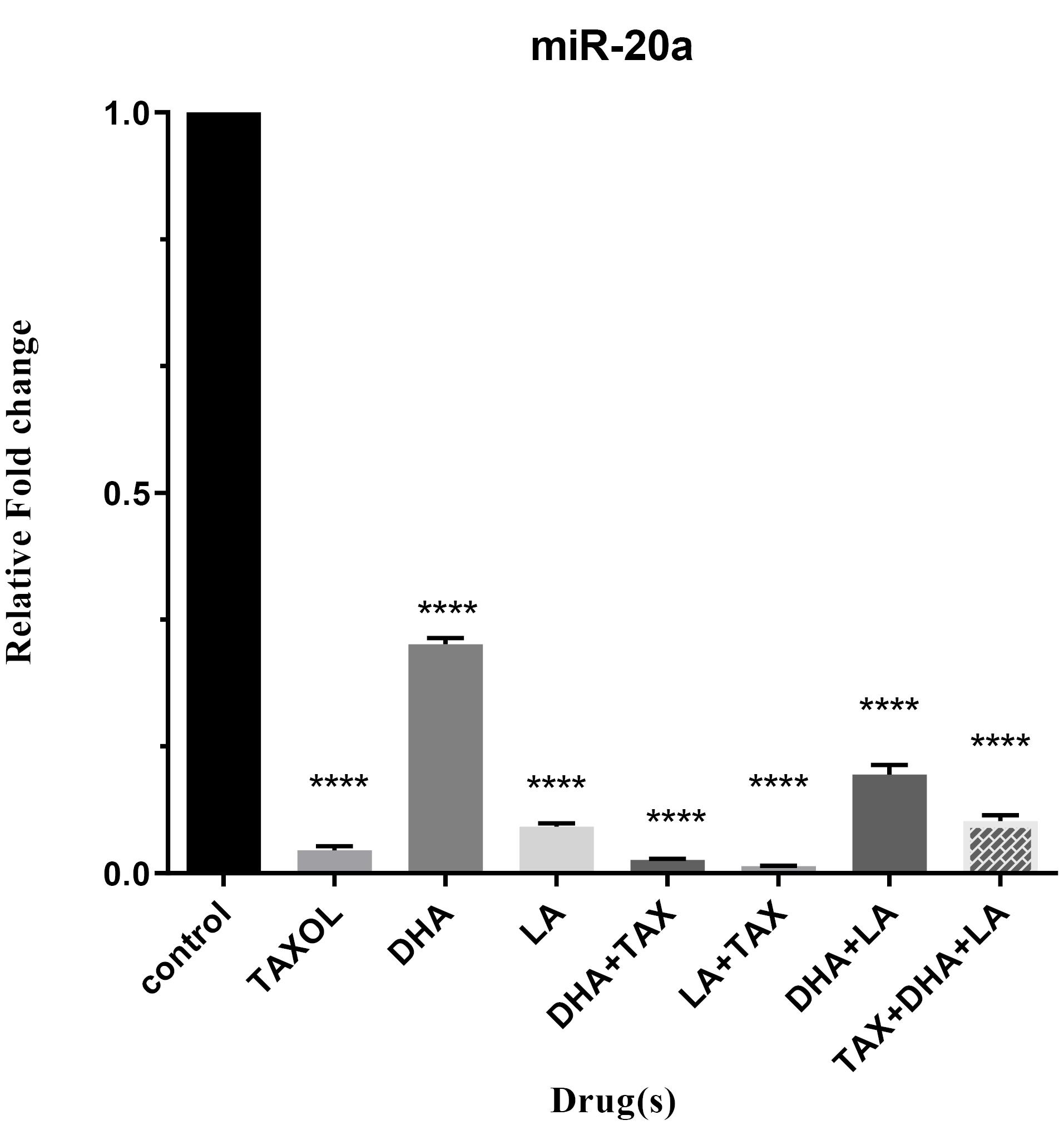
Figure 3.
Expression level of miR-20a in triple-negative metastatic human breast cancer cell line, MDA-MB-231, treated with DHA, LA, Taxol and their different combinations
.
Expression level of miR-20a in triple-negative metastatic human breast cancer cell line, MDA-MB-231, treated with DHA, LA, Taxol and their different combinations
Table 2.
The fold changes and P values of miR-10b, miR-20a and MICA
|
Treatment
|
miR-10b
|
miR-20a
|
MICA
|
|
Fold change
|
P
value
|
Fold change
|
P
value
|
Fold change
|
P
value
|
| Taxol |
-26.52 |
< 0.0001 |
-38.02 |
< 0.0001 |
-151.51 |
< 0.0001 |
| DHA |
-2.6 |
0.0115 |
-3.76 |
< 0.0001 |
-156.25 |
< 0.0001 |
| LA |
-5.91 |
< 0.0001 |
-18.72 |
< 0.0001 |
-196.07 |
< 0.0001 |
| DHA + TAX |
-7.68 |
< 0.0001 |
-64.51 |
< 0.0001 |
-78.74 |
< 0.0001 |
| LA + TAX |
-46.72 |
< 0.0001 |
-128.2 |
< 0.0001 |
-4.69 |
< 0.0001 |
| DHA + LA |
-2.92 |
< 0.0001 |
-7.88 |
< 0.0001 |
-161.29 |
< 0.0001 |
| TAX + DHA + LA |
-2.94 |
< 0.0001 |
-14.43 |
< 0.0001 |
-204.08 |
< 0.0001 |
DHA, LA, Taxol, and their combinations also decreased the level expression of the MICA in MDA-MB-231 cell line
The single most remarkable observation to emerge from the data comparison was, the reduced expression of MICA in all different combinations (P < 0.0001). In fact, contrary to what was previously thought, we found that treatment of BC cell lines with anti-cancer drugs accounted for lessening the expression of MICA. Contrary to expectations, we did not find a surprising difference in minimizing expression of MICA when treated with LA + Taxol combination compared with declining notably the expression of oncomiRs in this combination (Figure 4, Table 2).
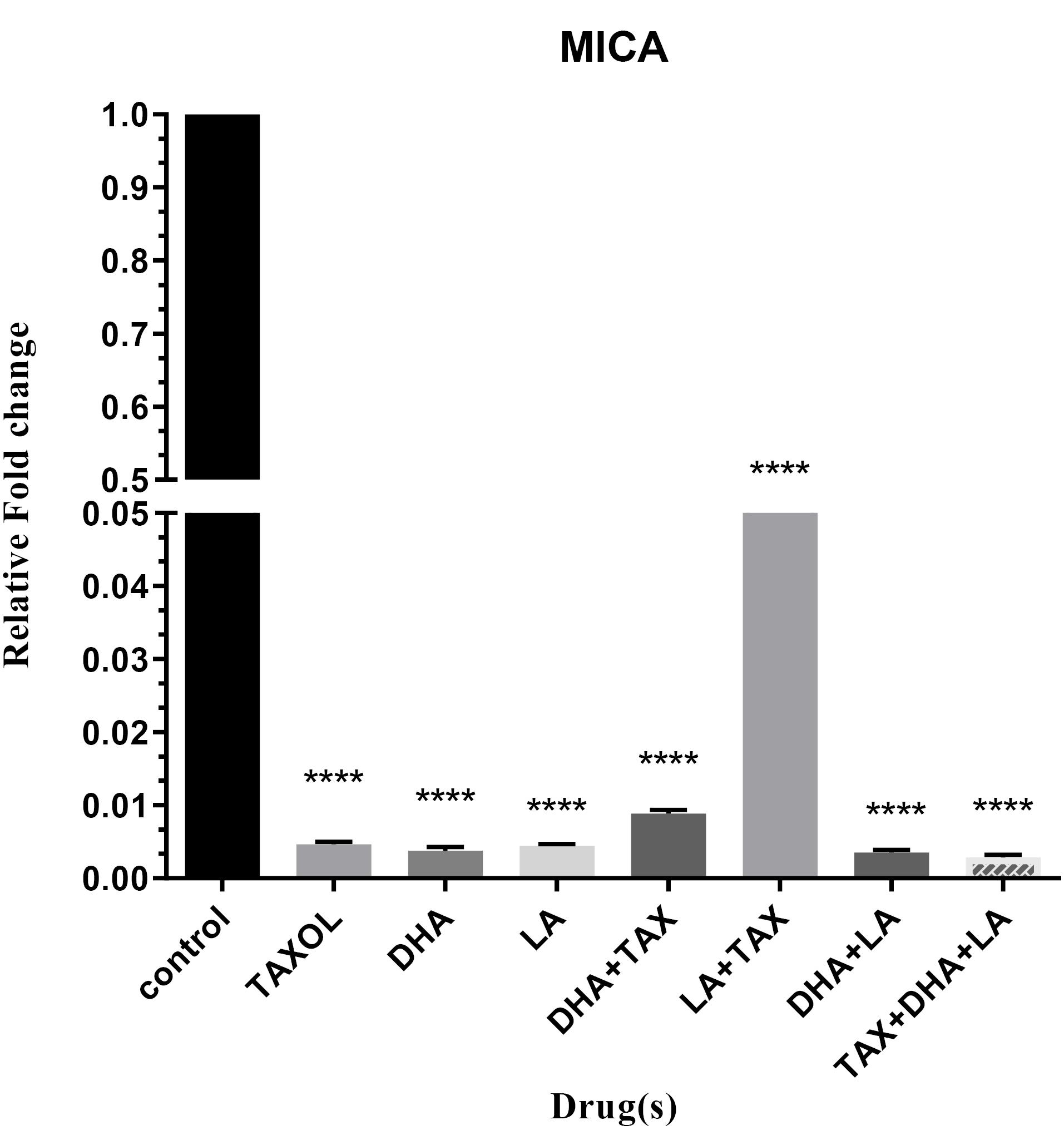
Figure 4.
Expression level of MICA in untreated and treated MDA-MB-231 cell lines with DHA, LA, Taxol and their different combinations
.
Expression level of MICA in untreated and treated MDA-MB-231 cell lines with DHA, LA, Taxol and their different combinations
Discussion
Due to the increasing of the cancer cases and considerable fatalities worldwide; much attention is paid to researches in the cancer field. Regarding BC, the ultimate goal of all efforts are to reach suitable therapy with lowest side effects. It has been indicated that DHA has very positive effects on the therapy of cancer along with the conventional therapeutics agents. However, molecular mechanisms of these effects particularly in the inhibition of the tumorigenesis are not clear.22 This study set out with the aim of assessing the effect of DHA, LA, and Taxol in declining miR-10b, miR-20a, and their target, MICA.
Several researches on various cancer types such as breast, ovarian, colorectal, lung, prostate, and gastric cancer have indicated that DHA (alone or in combination with the chemotherapeutic drugs) could raise tumor cell death through increasing apoptosis both in vivo and in vitro. Besides, researchers have found a distinct relation between DHA and invasion and also metastasis of tumor cells which could have a negative or positive effect on these processes.22 The present findings about the cytotoxicity effects of DHA on MDA-MB-231 cell lines seem to be consistent with other research which found DHA induces pyroptosis, which is an inflammation-related cell death in following cell lines.23 Another study which is done by Corsetto et al determined that DHA promoted apoptosis in MDA-MB-231 and MCF-7 cell lines by decreasing Bcl-2 and caspase-8 expression.24 Yun et al indicated that DHA has the ability of inhibiting cell proliferation and increasing of apoptosis through suppressing Wnt/β-catenin signaling pathways in the MDA-MB-231 BC cells, in a time and dose-dependent manner.25 About the synergic effects of DHA and Taxol, Li et al reported that DHA caused increasing sensitivity of BC cells and tumor-associated macrophages to Taxol.26 Wang et al. showed that the Taxol-DHA- carboxymethyl dextran drug delivery system, which conjugated S with containing amino acids Gly-Gly linkers removed xenograft tumors in nude mice-bearing MCF-7 BC cells.27 Shekari et al showed that DHA led to more down-regulation effect on MICA expression compared with combination treatment of DHA and docetaxel.28 Dong et al designed a drug conjugate with DHA, dextran, and docetaxel that had elimination effect on xenograft BC tumor in nude mice.29
Recent findings regarding the effects of LA in BC have led to contrary opinions in this area. In vivo and in vitro researches suggest that n-3 PUFAs may have anti-tumor impacts but about n-6 PUFAs vice versa effects have reported.30-34 Studies in BC showed that LA was contributor to rise rate of chronic diseases by induction of unsuitable inflammatory responses.35,36 In addition, LA have led to induction of plasminogen activator inhibitor-1 expression, proliferation, invasion, and migration of BC cells. Besides, EMT-like process has also observed in MCF10A through LA.37-39 Also, Marquez et alrevealed that LA increases MDA-MB-231 migration and invasion through PI3 kinases/Akt signaling pathway.40 However, Biatek et al concluded that LA accounts for suppressing BC in rat models of this disease.41 Likewise, a meta-analysis study noted that LA diminishes the risk of BC.42 It has been reported that, Taxol-LA loaded in a drug delivery system increased proliferation suppression of Non-Small Cell Lung Carcinoma compared to Taxol alone.43 In other study, it is indicated that treated gastric cancer cells with LA alone, and combination with docetaxel significantly decrease expression of miR-20a in docetaxel and LA + docetaxel while increasing expression seen in LA treatment.44
The most interesting finding in this study was the decrease of MICA in all treatments with anti-cancer drugs. Previous findings have reported that MICA is one of the ligands for NKG2D receptors which activates NK cells and causes anti-tumor responses.45-47 Induction of the NKG2DL expression occurs in neoplasm transformation and during stress conditions, while its expression is undetectable or low in normal cells.48 However, tumor cells are inhibited from recognition by immune cells by decreasing their surface expression of NKG2DLs.49,50 About BC contrary results have been demonstrated. In early BC patients, reported that high expression of MICA/B was an indicator of good prognosis51 whereas, in another study was shown higher MICA expression was found in BC tissues than in normal breast tissues.52 Also, Madjd et al proved that over expression of MICA was seen in poor prognosis of BC.53 In a Meta-analysis study, the higher soluble isoform of MICA/B (sMICA/B) was associated with a significantly lower overall survival (OS) rate in various cancer types.54 Kshersagar et al showed that sMICA in urine, saliva, and serum of BC patients were significantly higher than in normal group.55 Our results have some similarities with Madjd and colleagues’ findings that upregulation of MICA was detected in a high grade of BC.53 Furthermore in our study, expression of MICA significantly decreased in all treatments that matched results of Shekari’s study which reported MICA significantly down-regulated in treated gastric cancer cells with docetaxel, LA and docetaxel + LA.44
New findings indicate that miRNAs are critical roles in regulation of tumor development as an onco-miRs or tumor suppressor miRs.56 Following the present results, previous studies have demonstrated that miR-10b and miR-20a targeted MICA/B.57,58 Shen et al confirmed that MICA/B are targeted via miR-20a and miR-20b 21. In others, studies proved that miR-20a suppresses the expression of MICA through binding 3’-UTR sites in human tumor cells like HeLa, 293T, DU145.59,60 Codo et al indicated that glioma cancer cells express miR-20a to escape immunosurveillance by targeting MICA.16 Tsukerman et al showed that miR-10b down-regulated MICB in DU145, MCF-7, and RKO cell lines.61 It was found that down-regulation of miR-10b and miR-20a resulted in a reduction of MICA whereas Tang et al62 found that decreasing expression of MICA overexpressed miR-20a in colorectal cancer cells. Youlin et al showed that repression of miR-20a by a chemical compound (pterostilbene) caused up-regulation of MICA in prostate cancer cells.63
The evidence from this study suggests that MICA and its controlling miRs expression reduced after treatment with Taxol, DHA, LA, and different their combination. MICA expression had lower expression in TAX + DHA + LA treatment compared to other treatment groups. Additionally, LA + TAX is more effective in decreasing the expression of oncomiRs.
Conclusion
Finally, our study demonstrated DHA and LA has anti-tumor effects on MDA-MB-231 BC cells and this finding leads to the importance of the usage of omega-3 and omega-6 supplementation in cancer therapy.
DHA, and LA can alter miRNA expression levels in order to change tumor cells gene expression and ameliorate the efficacy of the chemotherapy. However, this effect is related to the therapeutic agent used and tumor cell line. Combination of these supplements with anti-cancer drug (Taxol) have had synergetic effect to decrease onco-miRs expression. Here, combined usage of LA and Taxol have more effect than other treatment groups. Further research should be done to investigate the level of MICA expression in the treatment of BC.
Acknowledgements
The authors wish to thank the financial support from the Kermanshah University of Medical Sciences, Kermanshah, Iran and Immunology Research Center, Tabriz University of Medical Science, Tabriz, Iran.
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
Not applicable.
References
- Wu J, Li M, Zhang Y, Cai Y, Zhao G. Molecular mechanism of activated T cells in breast cancer. Onco Targets Ther 2018; 11:5015-24. doi: 10.2147/ott.s173018 [Crossref] [ Google Scholar]
- Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015; 163(2):506-19. doi: 10.1016/j.cell.2015.09.033 [Crossref] [ Google Scholar]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490(7418):61-70. doi: 10.1038/nature11412 [Crossref] [ Google Scholar]
- Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013; 18(2):123-33. doi: 10.1634/theoncologist.2012-0397 [Crossref] [ Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121(7):2750-67. doi: 10.1172/jci45014 [Crossref] [ Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363(20):1938-48. doi: 10.1056/NEJMra1001389 [Crossref] [ Google Scholar]
- Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013; 346:f3706. doi: 10.1136/bmj.f3706 [Crossref] [ Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002; 56(8):365-79. doi: 10.1016/s0753-3322(02)00253-6 [Crossref] [ Google Scholar]
- Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer 2004; 111(4):584-91. doi: 10.1002/ijc.20284 [Crossref] [ Google Scholar]
- Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer 2003; 89(9):1672-85. doi: 10.1038/sj.bjc.6601314 [Crossref] [ Google Scholar]
- Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal?. Lipids Health Dis 2009; 8:33. doi: 10.1186/1476-511x-8-33 [Crossref] [ Google Scholar]
- Zou Z, Bidu C, Bellenger S, Narce M, Bellenger J. n-3 polyunsaturated fatty acids and HER2-positive breast cancer: interest of the fat-1 transgenic mouse model over conventional dietary supplementation. Biochimie 2014; 96:22-7. doi: 10.1016/j.biochi.2013.08.021 [Crossref] [ Google Scholar]
- Murray M. ω-3 Polyunsaturated fatty acids and their metabolites as inhibitors of mammalian tumorigenesis. Phytochem Rev 2014; 13(1):139-56. doi: 10.1007/s11101-013-9294-4 [Crossref] [ Google Scholar]
- Elieh Ali Komi D, Shekari N, Soofian-Kordkandi P, Javadian M, Shanehbandi D, Baradaran B. Docosahexaenoic acid (DHA) and linoleic acid (LA) modulate the expression of breast cancer involved miRNAs in MDA-MB-231 cell line. Clin Nutr ESPEN 2021; 46:477-83. doi: 10.1016/j.clnesp.2021.09.006 [Crossref] [ Google Scholar]
- Malumbres M. miRNAs and cancer: an epigenetics view. Mol Aspects Med 2013; 34(4):863-74. doi: 10.1016/j.mam.2012.06.005 [Crossref] [ Google Scholar]
- Codo P, Weller M, Meister G, Szabo E, Steinle A, Wolter M. MicroRNA-mediated down-regulation of NKG2D ligands contributes to glioma immune escape. Oncotarget 2014; 5(17):7651-62. doi: 10.18632/oncotarget.2287 [Crossref] [ Google Scholar]
- Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 2008; 9(9):1065-73. doi: 10.1038/ni.1642 [Crossref] [ Google Scholar]
- Bai X, Han G, Liu Y, Jiang H, He Q. MiRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3. Biomed Pharmacother 2018; 103:1482-9. doi: 10.1016/j.biopha.2018.04.165 [Crossref] [ Google Scholar]
- Crudele F, Bianchi N, Reali E, Galasso M, Agnoletto C, Volinia S. The network of non-coding RNAs and their molecular targets in breast cancer. Mol Cancer 2020; 19(1):61. doi: 10.1186/s12943-020-01181-x [Crossref] [ Google Scholar]
- Ma L. Role of miR-10b in breast cancer metastasis. Breast Cancer Res 2010; 12(5):210. doi: 10.1186/bcr2720 [Crossref] [ Google Scholar]
- Shen J, Pan J, Du C, Si W, Yao M, Xu L. Silencing NKG2D ligand-targeting miRNAs enhances natural killer cell-mediated cytotoxicity in breast cancer. Cell Death Dis 2017; 8(4):e2740. doi: 10.1038/cddis.2017.158 [Crossref] [ Google Scholar]
- Shekari N, Javadian M, Ghasemi M, Baradaran B, Darabi M, Kazemi T. Synergistic beneficial effect of docosahexaenoic acid (DHA) and docetaxel on the expression level of matrix metalloproteinase-2 (MMP-2) and microRNA-106b in gastric cancer. J Gastrointest Cancer 2020; 51(1):70-5. doi: 10.1007/s12029-019-00205-0 [Crossref] [ Google Scholar]
- Pizato N, Luzete BC, Kiffer L, Corrêa LH, de Oliveira Santos I, Assumpção JAF. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep 2018; 8(1):1952. doi: 10.1038/s41598-018-20422-0 [Crossref] [ Google Scholar]
- Corsetto PA, Montorfano G, Zava S, Jovenitti IE, Cremona A, Berra B. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis 2011; 10:73. doi: 10.1186/1476-511x-10-73 [Crossref] [ Google Scholar]
- Yun EJ, Song KS, Shin S, Kim S, Heo JY, Kweon GR. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix-metalloproteinases. Oncotarget 2016; 7(31):49961-71. doi: 10.18632/oncotarget.10266 [Crossref] [ Google Scholar]
- Li B, Tan T, Chu W, Zhang Y, Ye Y, Wang S. Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Deliv 2022; 29(1):75-88. doi: 10.1080/10717544.2021.2018523 [Crossref] [ Google Scholar]
- Wang S, Liu J, Lv H, Huang X, Dong P, Wang Q. Complete regression of xenografted breast tumors by dextran-based dual drug conjugates containing paclitaxel and docosahexaenoic acid. Eur J Med Chem 2022; 240:114567. doi: 10.1016/j.ejmech.2022.114567 [Crossref] [ Google Scholar]
- Shekari N, Javadian M, Ghaffari S, Baradaran B, Darabi M, Kazemi T. DHA abolishes the detrimental effect of docetaxel on downregulation of the MICA via decreasing the expression level of microRNA-20a in gastric cancer. J Gastrointest Cancer 2020; 51(2):545-51. doi: 10.1007/s12029-019-00280-3 [Crossref] [ Google Scholar]
- Dong P, Liu J, Lv H, Wu J, Zhang N, Wang S. The enhanced antitumor activity of the polymeric conjugate covalently coupled with docetaxel and docosahexaenoic acid. Biomater Sci 2022; 10(13):3454-65. doi: 10.1039/d2bm00337f [Crossref] [ Google Scholar]
- Bagga D, Anders KH, Wang HJ, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer 2002; 42(2):180-5. doi: 10.1207/s15327914nc422_5 [Crossref] [ Google Scholar]
- Chajès V, Torres-Mejía G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P. ω-3 and ω-6 Polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev 2012; 21(2):319-26. doi: 10.1158/1055-9965.epi-11-0896 [Crossref] [ Google Scholar]
- Rose DP, Connolly JM, Rayburn J, Coleman M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 1995; 87(8):587-92. doi: 10.1093/jnci/87.8.587 [Crossref] [ Google Scholar]
- Leslie MA, Abdelmagid SA, Perez K, Muller WJ, Ma DW. Mammary tumour development is dose-dependently inhibited by n-3 polyunsaturated fatty acids in the MMTV-neu(ndl)-YD5 transgenic mouse model. Lipids Health Dis 2014; 13:96. doi: 10.1186/1476-511x-13-96 [Crossref] [ Google Scholar]
- Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr 2007; 137(3):548-53. doi: 10.1093/jn/137.3.548 [Crossref] [ Google Scholar]
- Fritsche KL. Too much linoleic acid promotes inflammation-doesn’t it?. Prostaglandins Leukot Essent Fatty Acids 2008; 79(3-5):173-5. doi: 10.1016/j.plefa.2008.09.019 [Crossref] [ Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids 2001; 36(9):1007-24. doi: 10.1007/s11745-001-0812-7 [Crossref] [ Google Scholar]
- Espinosa-Neira R, Mejia-Rangel J, Cortes-Reynosa P, Salazar EP. Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol 2011; 43(12):1782-91. doi: 10.1016/j.biocel.2011.08.017 [Crossref] [ Google Scholar]
- Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y. Unsaturated fatty acids promote proliferation via ERK1/2 and Akt pathway in bovine mammary epithelial cells. Biochem Biophys Res Commun 2008; 367(4):729-35. doi: 10.1016/j.bbrc.2007.12.190 [Crossref] [ Google Scholar]
- Byon CH, Hardy RW, Ren C, Ponnazhagan S, Welch DR, McDonald JM. Free fatty acids enhance breast cancer cell migration through plasminogen activator inhibitor-1 and SMAD4. Lab Invest 2009; 89(11):1221-8. doi: 10.1038/labinvest.2009.97 [Crossref] [ Google Scholar]
- Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol 2017; 34(6):111. doi: 10.1007/s12032-017-0969-3 [Crossref] [ Google Scholar]
- Białek A, Tokarz A, Zagrodzki P. Conjugated linoleic acids (CLA) decrease the breast cancer risk in DMBA-treated rats. Acta Pol Pharm 2016; 73(2):315-27. [ Google Scholar]
- Zhou Y, Wang T, Zhai S, Li W, Meng Q. Linoleic acid and breast cancer risk: a meta-analysis. Public Health Nutr 2016; 19(8):1457-63. doi: 10.1017/s136898001500289x [Crossref] [ Google Scholar]
- Ngema LM, Adeyemi SA, Marimuthu T, Ubanako P, Wamwangi D, Choonara YE. Synthesis of novel conjugated linoleic acid (CLA)-coated superparamagnetic iron oxide nanoparticles (SPIONs) for the delivery of paclitaxel with enhanced in vitro anti-proliferative activity on A549 lung cancer cells. Pharmaceutics 2022; 14(4):829. doi: 10.3390/pharmaceutics14040829 [Crossref] [ Google Scholar]
- Shekari N, Kazemi T, Moradi M, Eghbali E, Sepehri B, Khaze Shahgoli V. Study of the effect of linoleic acid on the expression level of microRNA-106b and microRNA-20a and their related target MHC class I chain-related protein a in docetaxel-treated gastric cancer cells. Middle East J Cancer 2021; 12(3):342-9. doi: 10.30476/mejc.2021.87303.1406 [Crossref] [ Google Scholar]
- Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol 2010; 184(2):902-11. doi: 10.4049/jimmunol.0903225 [Crossref] [ Google Scholar]
- Unni AM, Bondar T, Medzhitov R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc Natl Acad Sci U S A 2008; 105(5):1686-91. doi: 10.1073/pnas.0701675105 [Crossref] [ Google Scholar]
- Smyth MJ, Swann J, Kelly JM, Cretney E, Yokoyama WM, Diefenbach A. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med 2004; 200(10):1325-35. doi: 10.1084/jem.20041522 [Crossref] [ Google Scholar]
- Pan J, Shen J, Si W, Du C, Chen D, Xu L. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 2017; 8(39):65743-58. doi: 10.18632/oncotarget.19445 [Crossref] [ Google Scholar]
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10(3):230-52. doi: 10.1038/cmi.2013.10 [Crossref] [ Google Scholar]
- Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature 2007; 447(7143):482-6. doi: 10.1038/nature05768 [Crossref] [ Google Scholar]
- de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer 2012; 12:24. doi: 10.1186/1471-2407-12-24 [Crossref] [ Google Scholar]
- Shen J, Pan J, Du C, Si W, Yao M, Xu L. Silencing NKG2D ligand-targeting miRNAs enhances natural killer cell-mediated cytotoxicity in breast cancer. Cell Death Dis 2017; 8(4):e2740. doi: 10.1038/cddis.2017.158 [Crossref] [ Google Scholar]
- Madjd Z, Spendlove I, Moss R, Bevin S, Pinder SE, Watson NF. Upregulation of MICA on high-grade invasive operable breast carcinoma. Cancer Immun 2007; 7(1):17. doi: 10.1158/1424-9634.dcl-17.7.1 [Crossref] [ Google Scholar]
- Zhao Y, Chen N, Yu Y, Zhou L, Niu C, Liu Y. Prognostic value of MICA/B in cancers: a systematic review and meta-analysis. Oncotarget 2017; 8(56):96384-95. doi: 10.18632/oncotarget.21466 [Crossref] [ Google Scholar]
- Kshersagar J, Damle MN, Bedge P, Jagdale R, Tardalkar K, Jadhav D. Downregulation of MICA/B tumor surface expressions and augmented soluble MICA serum levels correlate with disease stage in breast cancer. Breast Dis 2022; 41(1):471-80. doi: 10.3233/bd-220023 [Crossref] [ Google Scholar]
- Shah NR, Chen H. MicroRNAs in pathogenesis of breast cancer: implications in diagnosis and treatment. World J Clin Oncol 2014; 5(2):48-60. doi: 10.5306/wjco.v5.i2.48 [Crossref] [ Google Scholar]
- Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma. Br J Cancer 2015; 112(1):112-21. doi: 10.1038/bjc.2014.547 [Crossref] [ Google Scholar]
- Xie J, Liu M, Li Y, Nie Y, Mi Q, Zhao S. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell Mol Immunol 2014; 11(5):495-502. doi: 10.1038/cmi.2014.30 [Crossref] [ Google Scholar]
- Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev 2013; 253(1):167-84. doi: 10.1111/imr.12050 [Crossref] [ Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86. doi: 10.1002/ijc.29210 [Crossref] [ Google Scholar]
- Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res 2012; 72(21):5463-72. doi: 10.1158/0008-5472.can-11-2671 [Crossref] [ Google Scholar]
- Tang S, Fu H, Xu Q, Zhou Y. miR-20a regulates sensitivity of colorectal cancer cells to NK cells by targeting MICA. Biosci Rep 2019; 39(7):BSR20180695. doi: 10.1042/bsr20180695 [Crossref] [ Google Scholar]
- Youlin K, Simin L, Jian K, Li Z. Inhibition of miR-20a by pterostilbene facilitates prostate cancer cells killed by NK cells via up-regulation of NKG2D ligands and TGF-β1down-regulation. Heliyon 2023; 9(4):e14957. doi: 10.1016/j.heliyon.2023.e14957 [Crossref] [ Google Scholar]