ImmunoAnalysis. 4:8.
doi: 10.34172/ia.4084
Original Article
Matrix Metallopeptidase 9 Expression in Tumoral and Marginal Tissues of Cholesteatoma
Yalda Jabbari Moghaddam Data curation, Project administration, Resources, 1, 2 
Masoud Naderpour Germi Conceptualization, Methodology, Supervision, 1, 2, * 
Shabnam Noei Alamdary Conceptualization, Methodology, Supervision, 1, * 
Milad Emami Data curation, 1, 2
Dariush Shanehbandi Project administration, Visualization, Writing – original draft, 2
Saiedeh Razi Soofiyani Writing – review & editing, 3
Author information:
1Department of ENT, Imam Reza Hospital, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Clinical Research development unit, Sina Educational, Research and treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Cholesteatoma, a serious, expanding otolaryngologic condition, is challenging to otolaryngologists worldwide. The purpose of the present study was to evaluate the expression of MMP-9 gene in cholesteatoma patients.
Methods:
In this study, 40 samples of tumor tissue and 40 samples as control of tumor margins of patients with cholesteatoma were examined. Quantitative real-time polymerase chain reaction (PCR) was used for detection of the MMP-9 gene expression levels in cholesteatoma and control group. expression changes were calculated by ΔCT formula and data were analyzed by GraphPad Prism 8.
Results:
MMP-9 expression level in case group was significantly higher than the control group (P=0.0011). ROC analysis for the MMP-9 gene expression level that discriminates Cholesteatoma tumoral tissue from healthy tissues revealed a sensitivity of 67.5 % and a specificity of 84.62% with an area under the curve (AUC) value of 0.75 (P<0.001).
Conclusion:
The results indicated that MMP-9 is overexpressed in cholesteatoma tumoral tissues group compared to the healthy tissues. Based on AUC value of RUC curve analysis of MMP-9 expression level could be helpful in diagnosis of tumoral tissue form healthy tissue.
Keywords: Cholesteatoma, Neoplasms, Metalloproteases
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cholesteatoma is a benign lesion that gradually spreads and causes erosion of the temporal bones. This erosion causes the breakdown of adjacent bone structures.1 This bone resorption will be the prelude to various complications.1,2 Bone tissue resorption may lead to hearing loss, semicircular canals, facial paralysis, and intracranial complications. Cholesteatoma is divided into hereditary and acquired. Although cholesteatoma can have different origins, it has the same cellular mechanism.3 In cholesteatoma, the cell proliferation is altered, which affects the balance in the aggressive growth of squamous cell mucosal cells. However, it is not yet clear whether this imbalance is related to the mechanisms and genes defects involved in controlling cellular proliferation, or to cytokines released from inflammatory cells.4
Matrix metalloproteinases (MMPs) are calcium-dependent zinc-containing endopeptidases. On the whole, the activity of MMPs is tightly controlled, because increasing their activity terminates the extracellular matrix and thus increases the invasion of the epithelium.5 Studies have shown that an obvious imbalance in the regulation of MMPs in cholesteatoma leads to the destruction of the extracellular matrix by regulating increased MMP gene expression and decreasing MMP inhibitors.6-8
MMP-9 is the only member of the MMPs family that can bind and digest collagen as the most important component of the basement membrane due to its ternary structure of fibronectin and devastates extracellular matrix proteins.9 Therefore, the expression changes of this gene can have important functions in changing their function.10 The different type of cells, such as neutrophils, fibroblast endothelial cells, progenitor cells, connective tissue cells, tumor cells, and parenchymal cells produce MMP-9.11 To evaluate the clinical value in the development of cholesteatoma, and its role in cholesteatom prediction and prognosis, diagnosis and treatment monitoring, this study compared the expression levels of MMP-9 between the cholesteatoma and healthy tissues around the lesions.
Methods
Study design
This study was a case-control study. Patients who were referred to the Sina Educational, Research and Treatment Center in Tabriz for cholesteatoma surgery were entered in this study. Patients with complete clinical data and those who hadn’t obtained pertinent diagnoses and treatments in other hospitals meet the inclusion criteria. Patients with carious otitis media, middle ear malignancy, pregnancy, lactation, other severe diseases or tumors, communication problems, or cognitive impairment, and cholesteatoma patients who received treatment previously were excluded from the study. After explaining the study and its aims to the participants, written consents were obtained for attaining a pathology sample from patients. The provisions of the Helsinki Statement were considered in the study.
Detection method for expression level of MMP-9
Prior to molecular analyses being carried out in the Immunology Research Center, tissue samples were kept in RNA Later reagent (Invitrogen, Carlsbad, CA) containers. The tissues were homogenized in the RiboEx total RNA extraction solution (GeneAll Biotechnology CO, LTD, Korea). RNA isolation was carried out in accordance with the manufacturer’s instructions. Agarose gel electrophoresis and Thermo Scientific’s NanoDrop 2000c device were used to determine the concentration and quality of the isolated RNA samples.
cDNA was synthesized using a first standard complementary DNA (cDNA) synthesis kit(Fermentas Life Sciences, Vilnius, Lithuania). cDNA syntheses were carried out using 3 µg of total RNA. Real-time qRT-PCR was carried out utilizing the Light Cycler 96 equipment. In Table 1, the primer sequences are listed. GAPDH was employed as, housekeeping gene. Data analysis was accomplished using the ΔCT formula.
Table 1.
The primer sequences of MMP-9 and GAPDH genes
|
Gene
|
Primer sequences
|
TM (°C)
|
|
MMP9
|
|
59 |
| Forward |
TTGACAGCGACAAGAAGTGG |
|
| Reverse |
GCCATTCACGTCGTCCTTAT |
|
|
GAPDH
|
|
59 |
| Forward |
CAAGATCATCAGCAATGCCT |
|
| Reverse |
GCCATCACGCCACAGTTTCC |
|
Statistical analysis
Data were entered and analyzed by GraphPad Prism 8. Each data set underwent descriptive column statistics, and the Kolmogorov-Smirnov test was applied to indicate how the data were distributed. Mean ± standard deviation (SD) was applied to display quantitative data and frequency was applied to display qualitative data. To compare the demographic data between cholesteatoma and control group we used independent t test and chi square test. To assess the difference between two group we used paired t test. A receiver operating characteristic (ROC) analysis was done to evaluate the potential of MMP-9 expression in discriminating cholesteatoma and healthy tissue. AUC, or the area under the ROC curve, was calculated for the analysis using a 95% confidence interval. The P values lower than 0.05 were considered significant.
Results
General information
In this study, 40 samples of tumor tissue and 40 samples of tumor margins of patients with cholesteatoma were examined. Demographic information of patient showed in Table 2. As can be seen there was no significant relationship between the demographic specifications and MMP9 expression.
Table 2.
Demographic information of the cases and controls and the association of the demographic data and cholesteatoma
|
Variable
|
Cholesteatoma cases
No. (%)
|
Control
No. (%)
|
P
value
|
| Gender |
Female/Male |
Female/Male |
0.541 |
| 18 (45)/22 (55) |
17/23 |
| Age |
> 40 |
> 40 |
0.032 |
| 21 (52.5) |
12 |
| Smoking history |
Positive |
Positive |
0.041 |
| 9 (22.5) |
2 |
| Family history |
Positive |
Positive |
0.048 |
| 5 (12.5) |
1 |
MMP-9 expression in cholesteatoma and non-cholesteatoma tissues
Real-time PCR was used to evaluate the changes in the MMP-9 mRNA expression between cholesteatomaand control groups.
The results showed that MMP-9 expression level was significantly higher in the cholesteatoma group compared to the control group (P = 0.0011) (Figure 1).
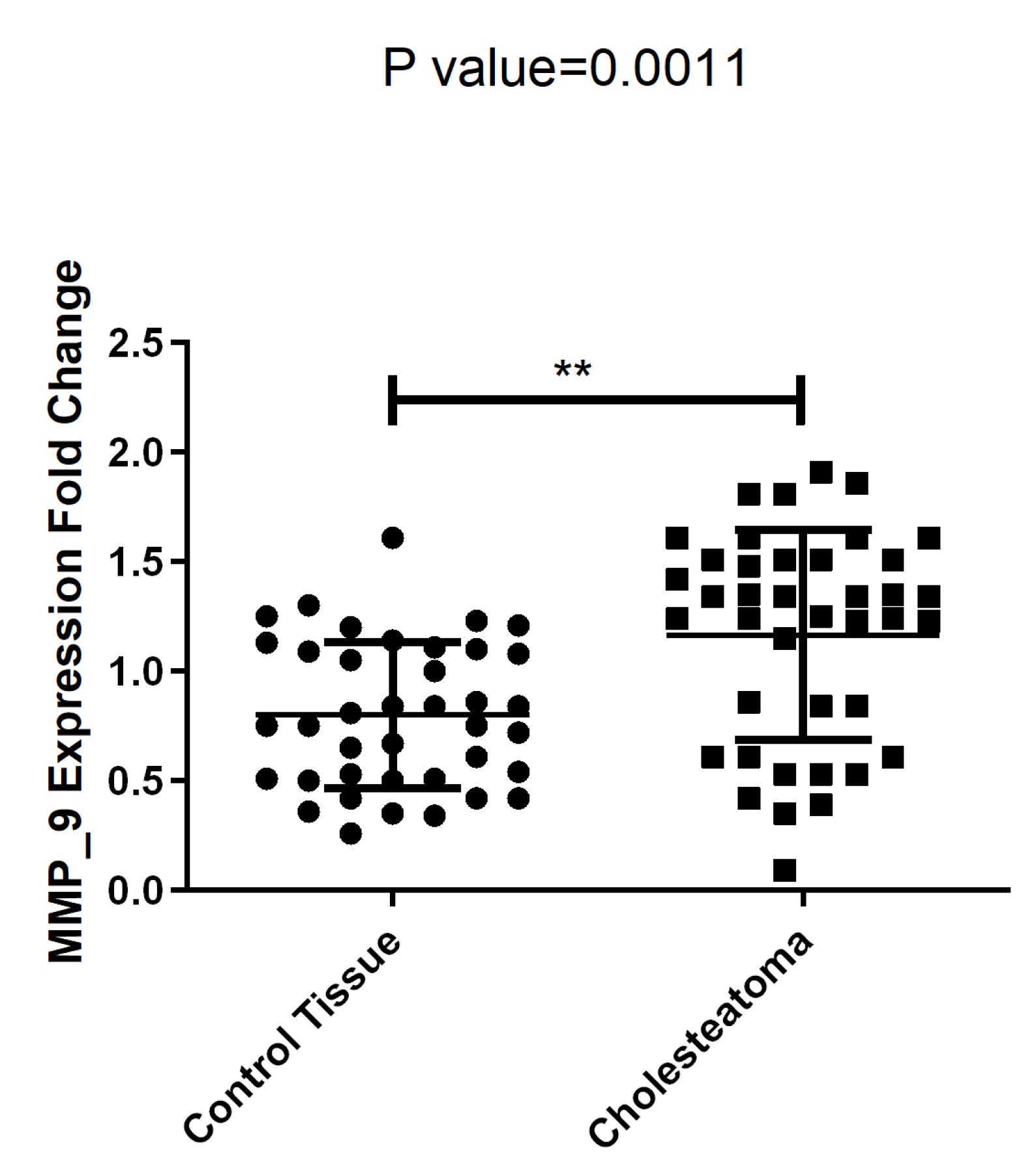
Figure 1.
MMP-9 expression in cholesteatoma tumoral tissues and control group. Note: **P value < 0.005
.
MMP-9 expression in cholesteatoma tumoral tissues and control group. Note: **P value < 0.005
MMP-9 expression and clinical significance in cholesteatoma
ROC curve plot was depicted according to MMP-9 expression to evaluate its diagnostic value in cholesteatoma. ROC analysis for the MMP-9 gene expression level that discriminates cholesteatoma tumoral tissue from healthy tissues revealed a sensitivity of 67.5 % and a specificity of 84.62% with an AUC value of 0.75 (P < 0.001) (Figure 2).
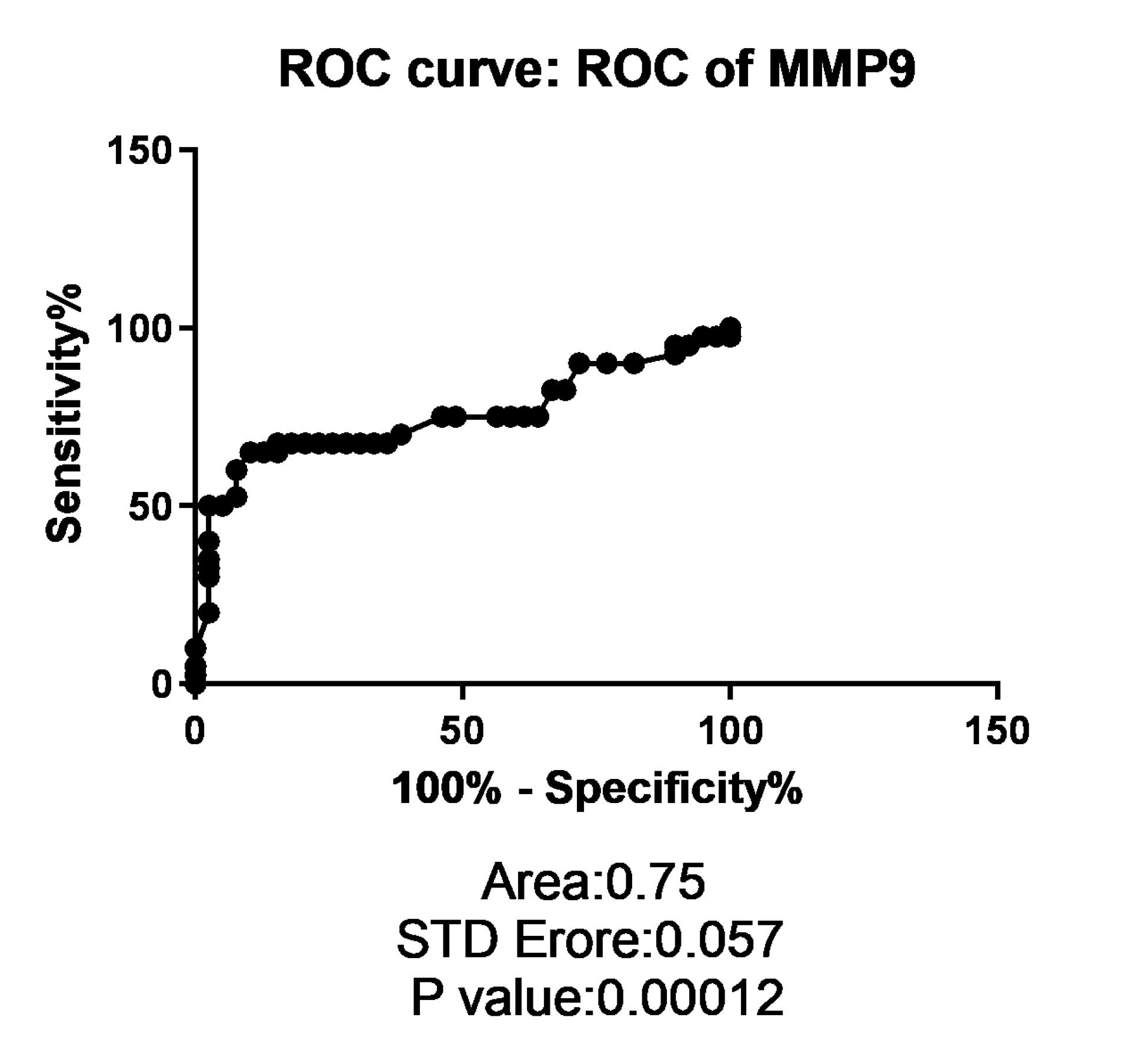
Figure 2.
ROC curve analysis. ROC curve shows AUCs of MMP-9, Sensitivity and specificity of MMP-9 expression in cholesteatoma
.
ROC curve analysis. ROC curve shows AUCs of MMP-9, Sensitivity and specificity of MMP-9 expression in cholesteatoma
Discussion
The aim of the present study was to analyze the expression level of the MMP-9 gene in patients with cholesteatoma. The results displayed that the expression level of MMP-9 in the tumoral tissue was significantly higher than in non-tumoral tissues.
In cholesteatoma the structures of the middle ear and mastoid bone is destroyed in an unpredictable way. Although cholesteatoma may be present for years, it is not clear what the mechanism of bone devastation is.12 Inflammation and inflammatory factors plays a main role in the cholesteatoma development and progression.13 For bone destruction to occur, the type I collagen in the matrix must first be broken down.14 The MMPs play an important role in the matrix and bone homeostasis as well as in osteolytic diseases. Also, MMPs are involved in the pathogenesis of malignant diseases.15
MMPs are enzymes that proteolize the extracellular matrix (ECM) inorganic contents. They are synthesized as pro-enzymes in most cases and secreted as pro-MMPs.16 MMPs are important both in the natural regeneration process and in the rapid degradation that occurs in wound healing,17 invasion of malignant tumors,18 and inflammatory diseases.19
The destruction of basement membrane and extracellular matrix in vivo and in vitro is associated with MMP-9 enzyme activity. The different type of cells such as epithelial cells, osteoblasts, fibroblasts, dendritic cells, keratinocytes, and T cells produce MMP-9.9 MMP-9 is involved in cellular migration, inflammatory process and the progressive activity of cholesteatoma cells.20 This metalloproteinase facilitates the release of angiogenic factors and thus increases the epithelial cells of the cholesteatoma.21 As the degree of damage to epithelial cells increases, the expression level of MMP-9 also increases, so MMP-9 is closely associated with cholesteatoma development and progression.22 Wu et al showed that, the expression level of MMP-9 in patients with progressive cholesteatoma is meaningfully more than in patients with localized ones, indicating the role of this metalloproteinase in the pathophysiology of the disease.23 The present study showed the increased expression of MMP-9 in cholesteatoma tumor cells than of the tumor margin cells.
Schmidt et al showed that MMP-9 expressed majorly in the super basal layers and to a lesser extent in the epithelium basal layers of the cholesteatoma like in pre-matrix inflammatory cells.24 Juhász et al study showed that MMP-9is overexpressed in the cholesteatoma and high level of MMP-9 expression is correlated with bone destruction in cholesteatoma progression process.25 In 2016, Olszewska et al found that MMP-9 expression level is higher in cholesteatoma tissues than its serum /plasma level in cholesteatomapatients.22 This study results indicate that the there is a strong association between high expression of MMP-9with inflammation in cholesteatoma.22 Wu et al in 2019 showed that overexpression of MMP-2/MMP-9 and IL-6 in the sera of patients with cholesteatoma’s otitis media are related to the bone damage degree.26 Also, Vranješ et al showed that overexpression of MMP-9, TNF-R2 and IL-1 was significantly associated with granulation and mucosal hypertrophy presence in the acquired middle ear cholesteatoma.27 Our study results were consistent with previous studies findings and confirmed them.
Conclusion
Our finding showed that the expression level of MMP-9 in the cholesteatoma group was significantly higher than the control group.
Based on AUC value of RUC curve analysis of MMP-9 expression level could be helpful in diagnosis of cholesteatoma by sensitivity of 67.5% and a specificity of 84.62%, which shows the clinical importance of evaluation of MMP-9 expression level in detection of cholesteatoma.
Acknowledgments
We would like to thank the Clinical Research Development Unit of Sina Educational, Research and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research and Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, for the financial support (grant number: 63229).
Competing Interests
The authors declared no competing interest.
Ethical Approval
The Ethics Committee of Tabriz University of Medical Sciences approved the conduction of this study (approval no: IR.TBZMED.REC.1400.1113, date: February 2, 2022).
References
- Venkatasamy R, Bee See G, Ismail F. Cholesteatoma morphed into temporal bone squamous cell carcinoma. Acta Otolaryngol Case Rep 2023; 8(1):152-6. doi: 10.1080/23772484.2023.2246645 [Crossref] [ Google Scholar]
- Rutkowska J, Özgirgin N, Olszewska E. Cholesteatoma definition and classification: a literature review. J Int Adv Otol 2017; 13(2):266-71. doi: 10.5152/iao.2017.3411 [Crossref] [ Google Scholar]
- Dorjee L. Comparison of Preoperative Temporal Bone HRCT Findings with Intraoperative Findings in Patients with Cholesteatoma [dissertation]. Madurai: Madurai Medical College; 2019.
- Dżaman K, Czerwaty K, Reichert TE, Szczepański MJ, Ludwig N. Expression and regulatory mechanisms of microRNA in cholesteatoma: a systematic review. Int J Mol Sci 2023; 24(15):12277. doi: 10.3390/ijms241512277 [Crossref] [ Google Scholar]
- Piperigkou Z, Manou D, Karamanou K, Theocharis AD. Strategies to target matrix metalloproteinases as therapeutic approach in cancer. Methods Mol Biol 2018; 1731:325-48. doi: 10.1007/978-1-4939-7595-2_27 [Crossref] [ Google Scholar]
- Naveed M, Han L, Hasnat M, Baig M, Wang W, Mikrani R. Suppression of TGP on myocardial remodeling by regulating the NF-κB pathway. Biomed Pharmacother 2018; 108:1460-8. doi: 10.1016/j.biopha.2018.09.168 [Crossref] [ Google Scholar]
- Tang N, Dong Y, Chen C, Zhao H. Anisodamine maintains the stability of intervertebral disc tissue by inhibiting the senescence of nucleus pulposus cells and degradation of extracellular matrix via interleukin-6/Janus kinases/signal transducer and activator of transcription 3 pathway. Front Pharmacol 2020; 11:519172. doi: 10.3389/fphar.2020.519172 [Crossref] [ Google Scholar]
- Mansour S, Magnan J, Nicolas K, Haidar H. Cholesteatoma. In: Middle Ear Diseases: Advances in Diagnosis and Management. Cham: Springer; 2018. p. 311-81. 10.1007/978-3-319-72962-6_8.
- Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem 2020; 194:112260. doi: 10.1016/j.ejmech.2020.112260 [Crossref] [ Google Scholar]
- Dofara SG, Chang SL, Diorio C. Gene polymorphisms and circulating levels of MMP-2 and MMP-9: a review of their role in breast cancer risk. Anticancer Res 2020; 40(7):3619-31. doi: 10.21873/anticanres.14351 [Crossref] [ Google Scholar]
- Barillari G. The impact of matrix metalloproteinase-9 on the sequential steps of the metastatic process. Int J Mol Sci 2020; 21(12):4526. doi: 10.3390/ijms21124526 [Crossref] [ Google Scholar]
- Stefanescu EH, Balica NC, Motoi SB, Grigorita L, Georgescu M, Iovanescu G. High-resolution computed tomography in middle ear cholesteatoma: how much do we need it?. Medicina (Kaunas) 2023; 59(10):1712. doi: 10.3390/medicina59101712 [Crossref] [ Google Scholar]
- Schürmann M, Oppel F, Shao S, Volland-Thurn V, Kaltschmidt C, Kaltschmidt B. Chronic inflammation of middle ear cholesteatoma promotes its recurrence via a paracrine mechanism. Cell Commun Signal 2021; 19(1):25. doi: 10.1186/s12964-020-00690-y [Crossref] [ Google Scholar]
- Kuo CL, Shiao AS, Yung M, Sakagami M, Sudhoff H, Wang CH. Updates and knowledge gaps in cholesteatoma research. Biomed Res Int 2015; 2015:854024. doi: 10.1155/2015/854024 [Crossref] [ Google Scholar]
- Paiva KB, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci 2017; 148:203-303. doi: 10.1016/bs.pmbts.2017.05.001 [Crossref] [ Google Scholar]
- Grakova EV, Shilov SN, Kopeva KV, Berezikova EN, Popova AA, Neupokoeva MN. Extracellular matrix remodeling in anthracycline-induced cardiotoxicity: what place on the pedestal?. Int J Cardiol 2022; 350:55-61. doi: 10.1016/j.ijcard.2022.01.013 [Crossref] [ Google Scholar]
- Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res 2017; 1864(11 Pt A):2015-25. doi: 10.1016/j.bbamcr.2017.05.007 [Crossref] [ Google Scholar]
- Fouad H, Salem H, Ellakwa DE, Abdel-Hamid M. MMP-2 and MMP-9 as prognostic markers for the early detection of urinary bladder cancer. J Biochem Mol Toxicol 2019; 33(4):e22275. doi: 10.1002/jbt.22275 [Crossref] [ Google Scholar]
- Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta Mol Cell Res 2017; 1864(11 Pt A):2036-42. doi: 10.1016/j.bbamcr.2017.05.010 [Crossref] [ Google Scholar]
- Castle JT. Cholesteatoma pearls: practical points and update. Head Neck Pathol 2018; 12(3):419-29. doi: 10.1007/s12105-018-0915-5 [Crossref] [ Google Scholar]
- Dambergs K, Sumeraga G, Pilmane M. Complex evaluation of tissue factors in pediatric cholesteatoma. Children (Basel) 2021; 8(10):926. doi: 10.3390/children8100926 [Crossref] [ Google Scholar]
- Olszewska E, Matulka M, Mroczko B, Pryczynicz A, Kemona A, Szmitkowski M. Diagnostic value of matrix metalloproteinase 9 and tissue inhibitor of matrix metalloproteinases 1 in cholesteatoma. Histol Histopathol 2016; 31(3):307-15. doi: 10.14670/hh-11-677 [Crossref] [ Google Scholar]
- Wu Y, Tang X, Shao W, Lu Y. Effect of CT manifestations of cholesteatoma on MMP-2, MMP-9 and IL-6 in the serum of patients. Exp Ther Med 2019; 17(6):4441-6. doi: 10.3892/etm.2019.7484 [Crossref] [ Google Scholar]
- Schmidt M, Grünsfelder P, Hoppe F. Up-regulation of matrix metalloprotease-9 in middle ear cholesteatoma--correlations with growth factor expression in vivo?. Eur Arch Otorhinolaryngol 2001; 258(9):472-6. doi: 10.1007/s004050100359 [Crossref] [ Google Scholar]
- Juhász A, Sziklai I, Rákosy Z, Ecsedi S, Adány R, Balázs M. Elevated level of tenascin and matrix metalloproteinase 9 correlates with the bone destruction capacity of cholesteatomas. Otol Neurotol 2009; 30(4):559-65. doi: 10.1097/MAO.0b013e31819fe6ed [Crossref] [ Google Scholar]
- Wu Y, Tang X, Shao W, Lu Y. Effect of CT manifestations of cholesteatoma on MMP-2, MMP-9 and IL-6 in the serum of patients. Exp Ther Med 2019; 17(6):4441-6. doi: 10.3892/etm.2019.7484 [Crossref] [ Google Scholar]
- Vranješ D, Špirić P, Gnjatić M. Expression of tumor necrosis factor alpha, interleukin-1 and matrix metalloproteinase-9 and pathomorphological changes in acquired middle ear cholesteatoma. Biomedicinska Istraživanja 2021; 12(1):29-38. doi: 10.5937/bii2101029v [Crossref] [ Google Scholar]