ImmunoAnalysis. 4:5.
doi: 10.34172/ia.4088
Original Article
Preferential Solvation of Cyclosporin in Aqueous Mixtures of Some Polymeric Cosolvents
María Ángeles Peña Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing, 1 
Olga E. García-Serna Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, 2
Darío A. Tinjacá Formal analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing, 3
Daniel R. Delgado Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, 4
Fleming Martínez Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 5, * 
Author information:
1Departamento de Ciencias Biomédicas, Facultad de Farmacia, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain
2Facultad de Ciencias de la Salud, Corporación Universitaria Adventista, Cra. 84 No. 33aa-01, Medellín, Colombia
3Universidad El Bosque, Facultad de Ciencias, Química Farmacéutica, Grupo de Investigación en Química aplicada (INQA), Semillero de investigación SIFUB, Av. Cra. 9 No. 131A-02, Bogotá D.C., Colombia
4Universidad Cooperativa de Colombia, Sede Neiva, Programa de Ingeniería Civil, Grupo de Investigación de Ingenierías UCC-Neiva, Facultad de Ingeniería, Neiva 410001, Colombia
5Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia –Sede Bogotá, Cra. 30 No. 45-03, Bogotá D.C., Colombia
Abstract
Background:
Cyclosporin is a cyclic peptide-drug used as immunosuppressant for the prophylaxisof transplant rejection whose physicochemical properties in mixed aqueous solvent systems isstill not well understood. The preferential solvation parameters of cyclosporin in aqueous binarymixtures of diethylene glycol monoethyl ether (DEGME), polyethylene glycol 200 (PEG 200) andpolyethylene glycol 400 (PEG 400) were computed.
Methods:
Reported mole fraction solubilities of cyclosporin in DEGME-aqueous mixtures,PEG 200-aqueous mixtures, and PEG 400-aqueous mixtures were processed by following theinverse Kirkwood-Buff integrals (IKBI) method as suggested by Marcus and Ben-Naim using somethermodynamic parameters reported in the literature for these aqueous-polymeric mixtures at298.15 K.
Results:
It is observed that cyclosporin is sensitive to preferential solvation effects in theseaqueous-polymeric binary solvent systems. The preferential solvation parameter by DEGME (δx1,3) is negative in water-rich mixtures but positive in mixtures of 0.12<x1<1.00. It is conjecturablethat hydrophobic hydration around the non-polar methyl and methylene groups of this drug thatcould be present in water-rich mixtures can significantly impact the drug solvation. Otherwise,in mixtures of 0.12<x1<1.00 in DEGME-aqueous mixtures, as well as in almost all the mixtureswith PEGs, the preferential solvation by polymeric cosolvents could be due to the acidic behaviorof cyclosporin in front of ether and hydroxyl oxygen atoms of these polymeric cosolvents.
Conclusion:
Cyclosporin is preferentially solvated by the polymeric solvents in almost all thestudied mixtures of these aqueous-polymeric binary solvent systems.
Keywords: Cyclosporin, (Polymeric solvent+water) mixtures, Inverse Kirkwood-Buff integrals, Preferential solvation
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This research was funded by the Research Directorate of the National University of Colombia with grant number HERMES 56224.
Introduction
Cyclosporin (condensed formula C62H111N11O12, molar mass 1202.64 g·mol−1, CAS Number: 59865-13-3, PubChem CID 5284373, molecular structure shown in Figure 1), is a lipophilic cyclic polypeptide of 11 amino acids produced by the fungus Beauveria nivea, also known asTolypocladium inflatum, is an immunosuppressant drug which binds with high affinity to an immunophilin termed cyclophilin. The complex cyclosporin-cyclophilin specifically inhibits calcineurin, a calcium- and calmodulin-dependent phosphatase distributed in all cellular compartments. The blockage of calcineurin prevents signal transduction of the nuclear factor of activated T-cells (NF-AT) which impairs gene transcription of interleukin (IL)-4 and CD40 ligand necessary for B-cell activation and those required for T-cell activation including IL-2 and interferon gamma. In this way, cyclosporin inhibits the first phase of T-cell activation leading to a reduced proliferation of T helper lymphocytes. It is also known that cyclosporin inhibits CD4 + CD25 + regulatory T cells, which may obstruct the ability for host immune tolerance.1-3
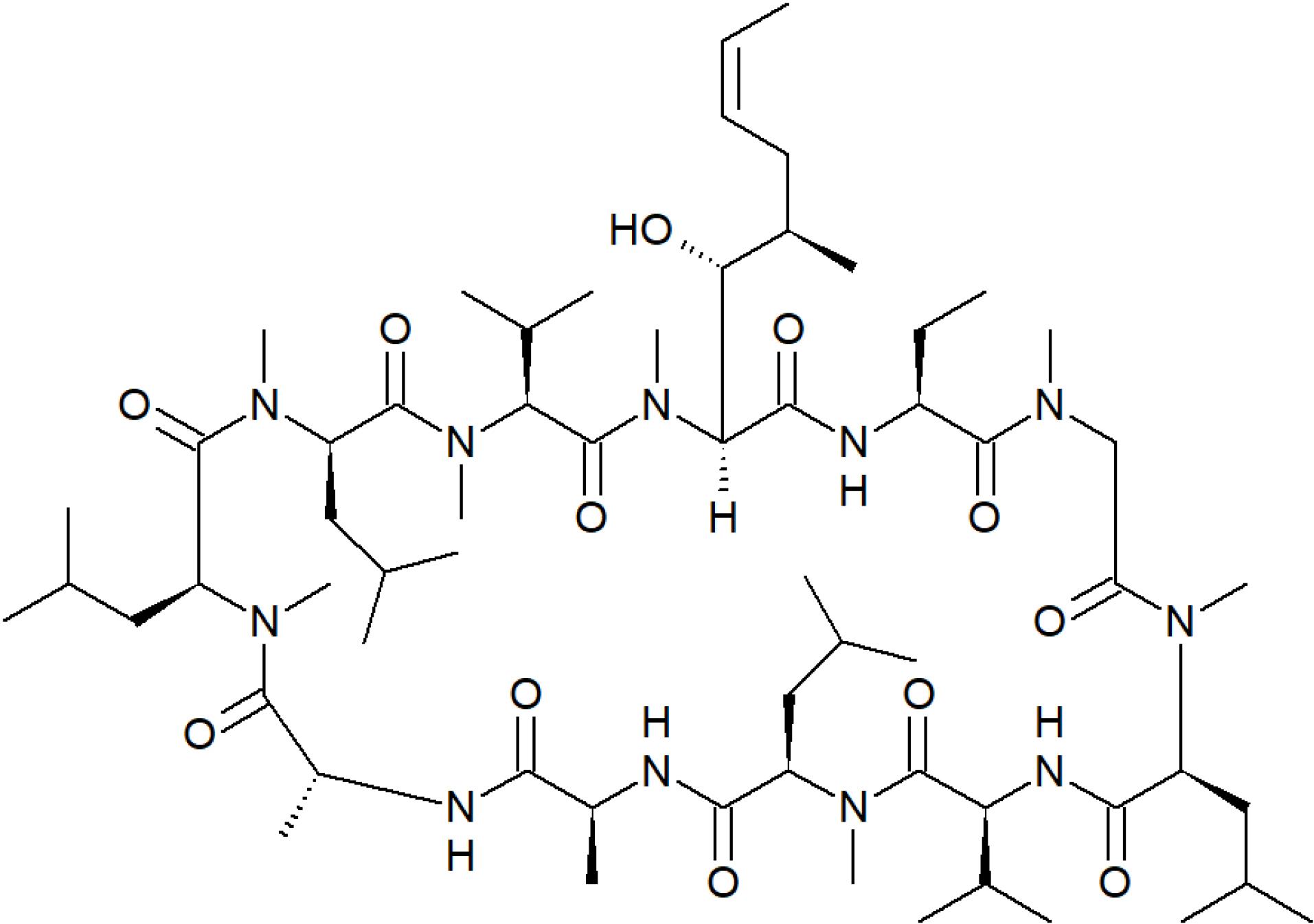
Figure 1.
Molecular structure of cyclosporin
.
Molecular structure of cyclosporin
Cyclosporin is used for the prophylaxis of transplant rejection, or in the treatment of graft rejection in patients previously treated with other immunosuppressants. It is also used in severe forms of immune diseases as uveitis, atopic dermatitis, psoriasis, amyotrophic lateral sclerosis, inflammatory bowel disease or rheumatoid arthritis when conventional therapy is ineffective or inappropriate. It is also useful in nephrotic syndrome and in other autoimmune component diseases like aplastic anemia, asthma, Behçet’s syndrome, chronic active hepatitis, multiple sclerosis, myasthenia gravis, polymyositis, and Kawasaki disease.4-6 Moreover, recently this drug was investigated for the treatment of COVID-19.7
From a solid-conglomerate point of view this drug is hydrophobic and the absorption can be affected by first-pass metabolism, mode of administration, formulation, and drug interactions. Its oral bioavailability ranges from 30% to 90% and exhibits a 95% lipoprotein bounding. Cyclosporin acts as a substrate and inhibitor of P-glycoprotein and it is metabolized by the CYP3A enzyme system in the liver, the gastrointestinal tract and kidney. Therefore, when cyclosporin is administered with inhibitors of both CYP3A4 and P-glycoprotein its bioavailability is improved leading to increased cyclosporin concentrations. In patients with normal hepatic function, the average half-life ranges from 16 to 27 hours, but can vary from 10-40 hours.3,8
Cyclosporin is available in several formulations: as an oral solution (100 mg/mL) or liquid-filled capsules (strength 25-50 and 100 mg); as an ophthalmic emulsion (strength 0.05%) and ophthalmic solution (strength 0.09% and 0.1%), and as a sterile solution (50 mg/mL) that must be diluted in 0.9% sodium chloride or 5% dextrose for intravenous slow infusion. The commercially available oral formulations of cyclosporin differ in their bioavailability, and patients should not be transferred from one to another without appropriate monitoring.3
The dosage and routes of administration depend on the indication. Intravenous route is preferred for kidney transplant reject prophylaxis, for treatment of graft-versus-host disease, acute severe ulcerative colitis, and Kawasaki disease. The oral route is used for other solid organ transplant reject prophylaxis and immune diseases. The ophthalmic route is used only for uveitis and keratoconjunctivitis.4,5
Adverse effects of cyclosporin are many and involves multiple vital organs. The most common is nephrotoxicity due to intense renal vasoconstriction, which reduces glomerular filtration rate. Hypertension and arrhythmia are also frequent. Metabolic and endocrinological adverse effects include hypertrichosis, hypomagnesaemia, hyperkalemia, dyslipidemia and gynecomastia. As cyclosporin may cause immunosuppression, patients are at increased risk of developing bacterial, viral, fungal, and protozoal infections. To reduce the most important adverse effects, monitoring serum levels of cyclosporin is mandatory. It is also important to check serum creatinine/BUN, serum bilirubin and serum electrolytes.4,5
Owing the low solubility of cyclosporin in neat water, for improving its apparent solubility several procedures have been proposed in the literature, as follows: solubilization with d-alphatocopheryl-polyethylene-glycol-1000 succinate9; solubilization by cosolvency with ethanol, propylene glycol, polyethylene glycol (PEG 400), glycofurol and glycerin, micellization with cremophor, tween 80 and tween 20, and complexation with α-cyclodextrin and hydroxypropyl β-cyclodextrin10; micellization with sodium cholate/lecithin-mixed micelles;11 complexation with mixed α-cyclodextrin and hydroxypropyl-β-cyclodextrin,12 liposomes and other heterogeneous systems13; self-microemulsifying systems14; polymeric nanospheres15; microspheres based on αβ-cyclodextrins polymers16; microfiber obtained by electrospinning17; liposomes and other colloidal systems18; and drug-loaded nanofibers.19 In particular, dosage forms intended for ophthalmic administration has specially studied.20-22
Regarding molecular dispersion-solubility studies of cyclosporin in water the investigations by Ismailos et al23 and Molpeceres et al24 demonstrated some non-common behaviors involving drug solubility diminishing with temperature-arising. More recently, Berton et al reported the cyclosporin solubility in six ionic liquids at 25 and 100 °C;25 whereas, Ha et al reported the solubility of this drug at 25.0 °C in 20 mono-solvents, namely, acetone, acetonitrile, 1-butanol, chloroform, diethylene glycol monoethyl ether (DEGME), dichloromethane, N,N-dimethylformamide, dimethyl sulfoxide, ethanol, glycerol, methanol, N-methyl-2-pyrrolidone, 1-propanol, 2-propanol, propylene glycol, PEG 200, PEG 300, PEG 400, tetrahydrofuran, and water, as well as in some aqueous-polymeric cosolvent mixtures.26
As indicated above the physicochemical information about cyclosporin dispersed at molecular level in multi-component solvent systems is not complete as required for optimum liquid pharmaceutical dosage forms design. From practical and theoretical points of view, the drug behavior in binary or ternary cosolvent mixtures is frequently studied for improving substances purification and pharmaceutical preformulation stages.27-30
Owing the low solubility of cyclosporin in neat water, some binary aqueous cosolvent mixtures, involving the following polymeric cosolvents: DEGME (also known as carbitol and transcutol), PEG 200, PEG 300 and PEG 400 have been studied to increase its solubility.26 It is important to note that DEGME, PEG 200 and PEG 400 are common polymeric cosolvents used for several liquid medicines including injectable products.31,32 For this reason, the main purposes of this communication were evaluation the effect of mixtures polarity on cyclosporine solubility as well as reporting the preferential solvation of cyclosporin by cosolvents and water in some {polymeric cosolvent + water} mixtures at 298.15 K based on literature solubility values and some thermodynamic properties by means of the inverse Kirkwood-Buff integrals (IKBI).33-35 The results are expressed in terms of changing the preferential solvation parameter (δx1,3) of cyclosporin (compound 3) by the respective cosolvent (component 1, i.e. polymeric cosolvent DEGME, PEG 200 or PEG 400) regarding the mixtures composition.
Methods and Computation
Physicochemical properties of cyclosporin dissolutions, involving cosolvency effects and preferential solvation, were calculated as reported earlier in the literature for other organic compounds in similar solvent mixtures as shown below.36,37 All computations were performed with MS Excel® and Table Curve 2D v.5.01.
Results and Discussion
Effect of polarity on the cyclosporin solubility in aqueous mixtures
Mole fraction solubility of cyclosporin in aqueous binary mixtures of polymeric cosolvents is reported in the research article written by Ha et al.26 Thus, Figure 2 allows the comparison of mole fraction solubilities of this drug in the aqueous mixtures of polymeric cosolvents DEGME, PEG 200 and PEG 400 as function of the Hildebrand solubility parameter of the mixtures free of drug (δ1 + 2/MPa1/2) at 298.15 K. It is noteworthy that δ1 + 2 values were calculated assuming additive behavior as shown in Eq. (1).27-29 Solubility parameters of DEGME, PEG 200, PEG 400 and water are 22.3, 21.6, 24.3 and 47.8 MPa1/2, respectively.38,39
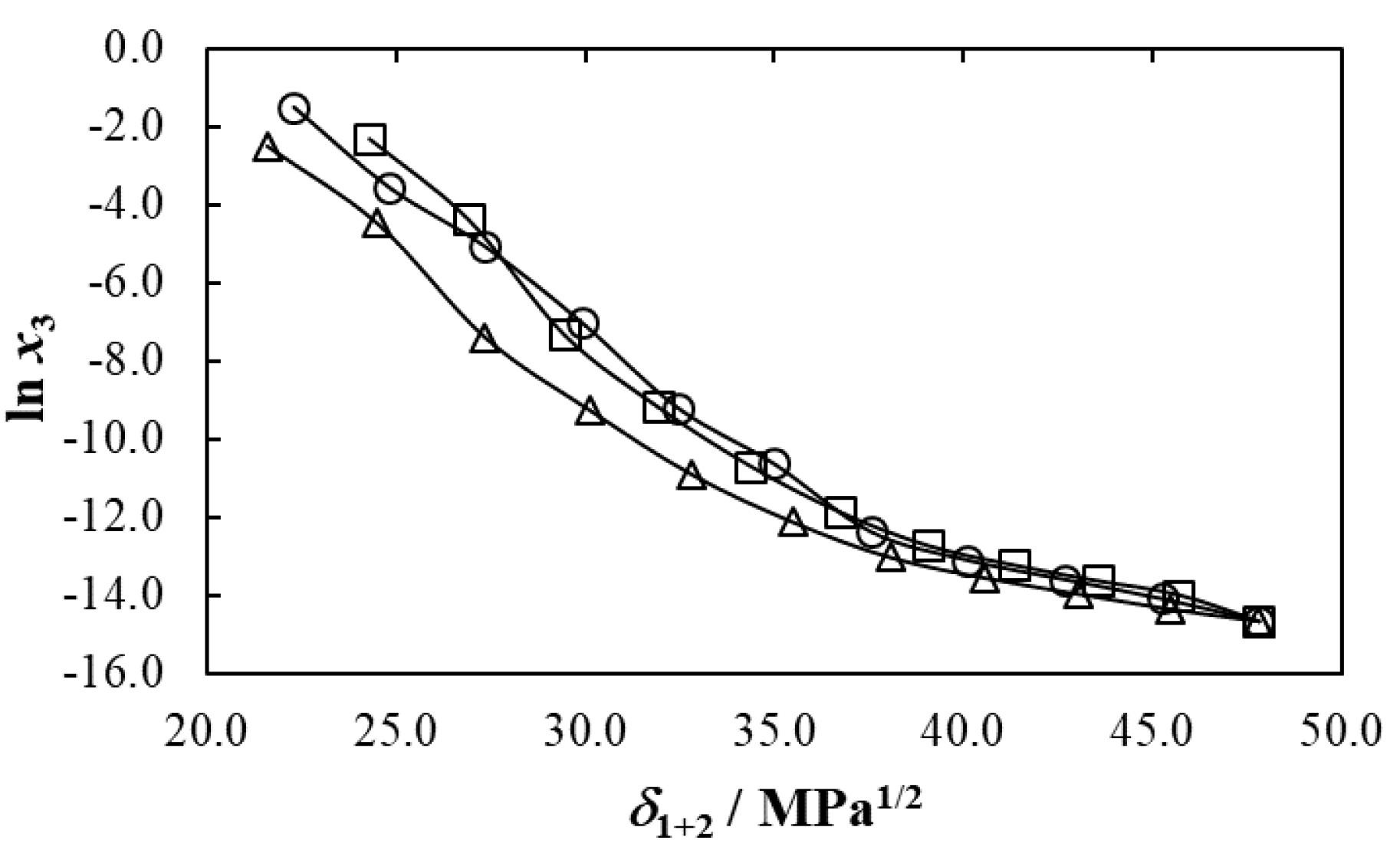
Figure 2.
Logarithmic mole fraction solubility of cyclosporin as function of the Hildebrand solubility parameter in some aqueous-polymeric cosolvent mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); Δ: PEG 400 (1) + water (2)
.
Logarithmic mole fraction solubility of cyclosporin as function of the Hildebrand solubility parameter in some aqueous-polymeric cosolvent mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); Δ: PEG 400 (1) + water (2)
As observed in all cases, the maximum solubility is observed in pure polymeric cosolvents. Moreover, cyclosporin solubility is similar in DEGME and PEG 200 mixtures and lower in aqueous-PEG 400 mixtures.
Table 1 summarizes the apparent Gibbs energies of dissolution at 298.15 K calculated by using Eq. (2).40 As observed all ∆solnG° are positive and diminish with the polymeric cosolvent proportion, regardless the cosolvent that demonstrates the cyclosporin preference by semi-polar media.
Table 1.
Apparent Gibbs energies of dissolution and transfer of cyclosporin in some {polymeric cosolvent (1) + water (2) mixtures at T = 298.15 K
|
w
1
|
DEGME+water
|
PEG 200+water
|
PEG 400+water
|
|
x
1
|
∆soln
G
° / kJ·mol–1
|
∆tr
G
° / kJ·mol–1
|
x
1
|
∆soln
G
° / kJ·mol–1
|
∆tr
G
° / kJ·mol–1
|
x
1
|
∆soln
G
° / kJ·mol–1
|
∆tr
G
° / kJ·mol–1
|
| 0.00 |
0.0000 |
36.35 |
0.00 |
0.0000 |
36.35 |
0.00 |
0.0000 |
36.35 |
0.00 |
| 0.10 |
0.0147 |
34.91 |
-1.43 |
0.0099 |
34.68 |
-1.67 |
0.0050 |
35.58 |
-0.76 |
| 0.20 |
0.0325 |
33.70 |
-2.65 |
0.0220 |
33.78 |
-2.57 |
0.0111 |
34.63 |
-1.72 |
| 0.30 |
0.0544 |
32.47 |
-3.88 |
0.0372 |
32.78 |
-3.57 |
0.0189 |
33.62 |
-2.73 |
| 0.40 |
0.0822 |
30.66 |
-5.68 |
0.0566 |
31.54 |
-4.80 |
0.0291 |
32.30 |
-4.04 |
| 0.50 |
0.1184 |
26.36 |
-9.99 |
0.0826 |
29.42 |
-6.92 |
0.0431 |
30.06 |
-6.29 |
| 0.60 |
0.1676 |
22.81 |
-13.54 |
0.1190 |
26.51 |
-9.84 |
0.0633 |
27.07 |
-9.28 |
| 0.70 |
0.2386 |
17.38 |
-18.97 |
0.1737 |
22.78 |
-13.56 |
0.0951 |
22.98 |
-13.36 |
| 0.80 |
0.3494 |
12.59 |
-23.75 |
0.2649 |
18.19 |
-18.16 |
0.1527 |
18.36 |
-17.99 |
| 0.90 |
0.5472 |
8.85 |
-27.50 |
0.4477 |
10.86 |
-25.48 |
0.2884 |
11.14 |
-25.21 |
| 1.00 |
1.0000 |
3.74 |
-32.60 |
1.0000 |
5.76 |
-30.58 |
1.0000 |
6.23 |
-30.12 |
w1 and x1 are the mass and mole fractions of polymeric cosolvent (1) in the {polymeric cosolvent (1) + water (2)} mixtures free of cyclosporin (3), respectively.
Preferential solvation of cyclosporin by mixtures components
The preferential solvation parameters of cyclosporin (identified here as compound 3) by polymeric cosolvent molecules, namely, DEGME, PEG 200 or PEG 400 (identified here as compound 1) molecules in the different {polymeric cosolvent (1) + water (2)} mixtures (δx1,3), are defined as33-35:
(3)
where
is the local mole fraction of polymeric cosolvent in the molecular environment of cyclosporin and x1 is the bulk mole fraction of polymeric cosolvent in the initial {polymeric cosolvent (1) + water (2)} binary solvent mixture free of cyclosporin. Thus, if δx1,3 value is positive cyclosporin molecules are preferentially solvated by polymeric cosolvent molecules in the respective dissolution. In contrast, cyclosporin molecules are preferentially solvated by water molecules if this δx1,3 parameter is negative. When |δx1,3| ≤ 0.01 the values are within the error of the determination that implies negligible preferential solvation. Complete selective solvation of cyclosporin (3) by polymeric solvent (1) takes place when δx1,3 ≈ x2, implying that δx1,3 cannot be larger than x2.33-35
The preferential solvation of cyclosporin (3) in the {polymeric cosolvent (1) + water (2)} mixture depends not only on the interactions of cyclosporin with polymeric solvent (1) and with water (2) but also on the mutual interactions of the two solvents as described by the molar excess Gibbs energy of their mixing in the absence of cyclosporin (3) (
). It is important to note that competitive interactions among all three components can take place in the solutions. The values of δx1,3 were obtained from the IKBI based on Ben-Naim equations as described earlier33-35:
(4)
with,
(5)
(6)
(7)
Here, κT denotes the isothermal compressibility of the (1 + 2) aqueous-polymeric cosolvent mixtures.
,
and
are respectively the partial molar volumes of polymeric cosolvent (DEGME, PEG 200 or PEG 400), water, and cyclosporin in the solutions. The function D as defined in Eq. (8) corresponds to the first derivative of the standard molar Gibbs energies of transfer of cyclosporin from neat water to every (1 + 2) aqueous-polymeric cosolvent mixture regarding the mole fraction of polymeric cosolvent. The function Q as defined in Eq. (9) involves the second derivative of the excess molar Gibbs energy of mixing of polymeric cosolvent (1) and water (2) (
) regarding the mole fraction of water (2).33-35 Vcor and r3 are respectively the correlation volume and the gyration molecular radius of cyclosporin. Here, r3 was roughly calculated by using Eq. (10), where NAv is the number of Avogadro, regardless the non-spherical of these drug molecules.41
(8)
(9)
(10)
To obtain definitive Vcor values some iteration processes were performed because they depend on the local mole fractions of polymeric cosolvent (1) and water (2) around the cyclosporin (2) molecules in the respective solutions. Thus, these iteration processes were performed by replacing δx1,3 and Vcor in equations (3), (4) and (7) to recalculate the
values until obtaining non-variant values of Vcor.
Table 2 and Figure 3 shows the apparent Gibbs energies of transfer of cyclosporin (3) from neat water (2) to all aqueous-polymeric cosolvent (1 + 2) mixtures (
) at 298.15 K. These
values were calculated from the experimental mole fraction solubility values reported in the Ha et al research article,26 by using Eq. (11).
values can also be calculated by using the ∆solnG° values reported in Table 1 of this work.
Table 2.
Coefficients and statistical parameters of equation (12) applied to Gibbs energies of transfer of cyclosporin from neat water (1) to some {polymeric solvent (1) + water (2)} mixtures at T= 298.15 K
|
Coefficient or statistical parameter
|
DEGME+water
|
PEG 200+water
|
PEG 400+water
|
|
a
|
-0.246 |
-0.007 |
-0.019 |
|
b
|
-2.818 |
1.500 × 103 |
8.121 × 102 |
|
c
|
-6.070 × 101 |
-1.317 × 103 |
-4.724 × 101 |
|
d
|
1.129 × 101 |
8.495 × 102 |
3.004 × 103 |
|
e
|
4.496 × 101 |
-1.151 × 105 |
-1.248 × 105 |
|
f
|
1.215 × 101 |
3.768 × 103 |
1.667 × 104 |
|
g
|
-6.888 × 102 |
-7.074 × 104 |
-4.924 × 105 |
| Adjusted r2 |
0.998 |
0.999 |
0.999 |
| Typical error |
0.476 |
0.298 |
0.322 |
|
F statistic |
957 |
1947 |
1717 |
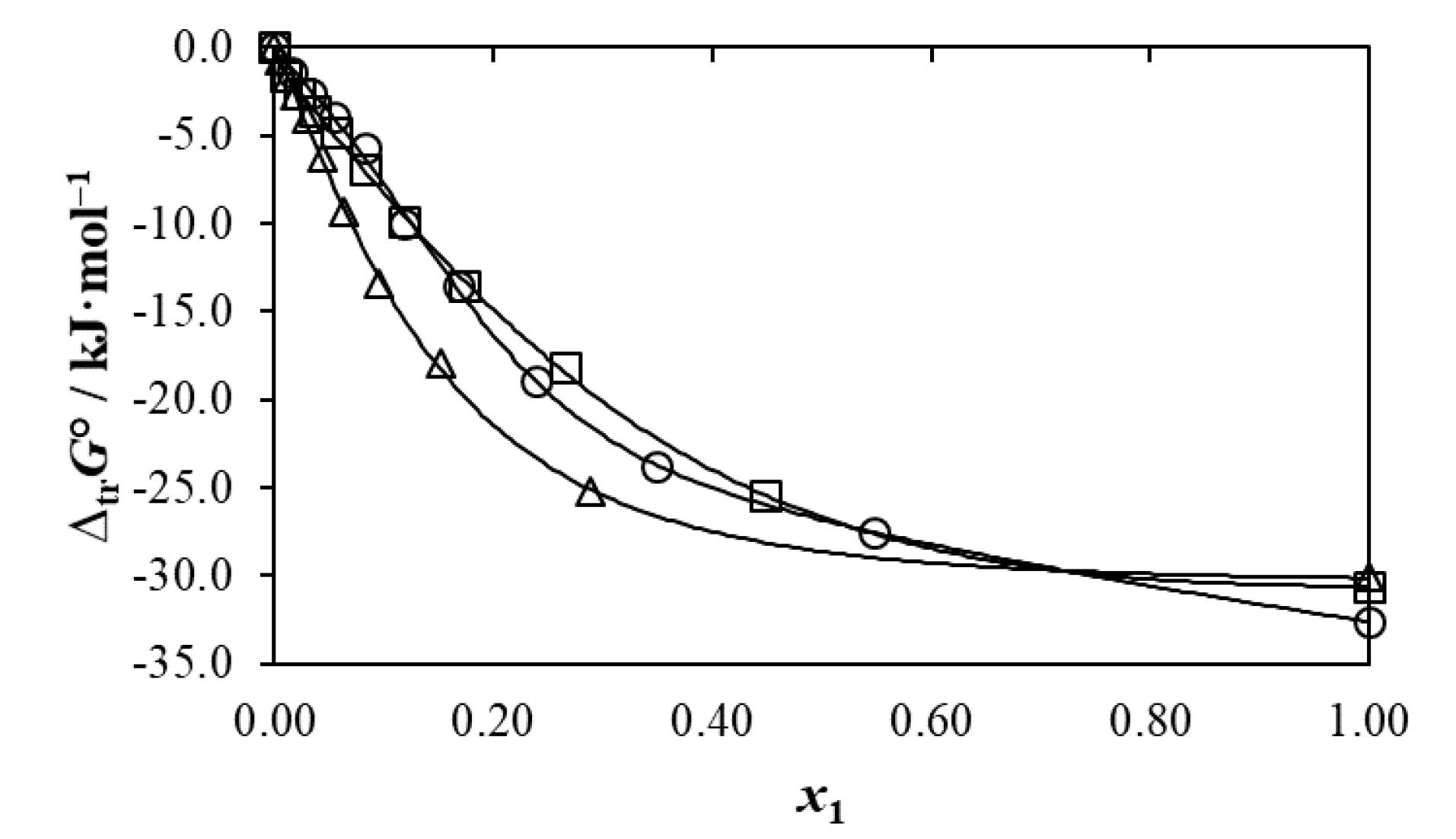
Figure 3.
Gibbs energy of transfer of cyclosporin (3) from neat water (2) to some {polymeric solvent (1) + water (2)} mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); ∆: PEG 400 (1) + water (2)
.
Gibbs energy of transfer of cyclosporin (3) from neat water (2) to some {polymeric solvent (1) + water (2)} mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); ∆: PEG 400 (1) + water (2)
(11)
It is important to keep in mind that cyclosporin–cyclosporin interactions may be disregarded and thus the cyclosporin (3) molecules are surrounded by polymeric cosolvent (1) and water (2) molecules only. Otherwise, activity coefficients of cyclosporin at each cosolvent mixture composition need to be employed. Another requirement for the application of Eq. (11) is that no crystal solvates are formed by cyclosporin, which means that the conglomerate identity of the cyclosporin solid form at equilibrium with the saturated solutions is independent of the cosolvent mixtures composition.
Obtained
values were correlated by using the quotient-polynomial shown as Eq. (12). Coefficients and statistical parameters obtained with Eq. (12) for all the aqueous-polymeric cosolvent systems are shown in Table 2.
(12)
The D values summarized in Table 3 were calculated as the first derivative of Eq. (12) solved in mixtures composition steps of x1 = 0.05. For the studied aqueous-polymeric cosolvent mixtures, the Q, RT·κT,
and
values at 298.15 K were taken from the literature as follows, for aqueous-DEGME mixtures,42 for aqueous-PEG 200 mixtures,43 and for aqueous-PEG 400 mixtures.44 In this research, the
value was considered as one calculated by using the groups contribution method proposed by Fedors, namely 935.8 cm3·mol–1,26,45 regardless the aqueous-polymeric cosolvent mixture under consideration. Table 3 shows that both the G1,3 and G2,3 values are negative in all the aqueous-polymeric cosolvent systems indicating the affinity of cyclosporin (3) for both solvents in the mixtures, polymeric cosolvent (1) and water (2). Cyclosporin r3 value was calculated as 0.719 nm. As indicated above, Vcor values shown in Table 3 were obtained after three iterations. Vcor values increase with the polymeric cosolvent-proportion in the mixtures because the
values are higher than the
values in all cases.46-48 Further, Table 3 additionally summarizes the preferential solvation parameters of cyclosporin by polymeric cosolvent molecules (δx1,3) in all these mixtures at 298.15 K.
Table 3.
Some properties associated to preferential solvation of cyclosporin (3) in some {polymeric cosolvent (1) + water (2)} mixtures at T = 298.15 K
|
x
1
|
D
/
kJ·mol–1
|
G
1,3
/ cm3
·mol–1
|
G
2,3 / cm3
·mol–1
|
V
cor
/ cm3
·mol–1
|
100
δ
x
1,3
|
| DEGME + water |
| 0.00 |
-61.39 |
-1381 |
-935 |
2455 |
0.00 |
| 0.05 |
-75.63 |
-1567 |
-1174 |
2647 |
-1.28 |
| 0.10 |
-87.64 |
-1715 |
-1569 |
2901 |
-1.00 |
| 0.15 |
-87.98 |
-1705 |
-1948 |
3263 |
2.29 |
| 0.20 |
-74.94 |
-1539 |
-2084 |
3586 |
5.41 |
| 0.25 |
-56.43 |
-1337 |
-1975 |
3802 |
6.02 |
| 0.30 |
-40.27 |
-1183 |
-1777 |
3962 |
5.28 |
| 0.35 |
-29.05 |
-1088 |
-1605 |
4111 |
4.37 |
| 0.40 |
-22.08 |
-1035 |
-1490 |
4264 |
3.69 |
| 0.45 |
-17.92 |
-1006 |
-1426 |
4422 |
3.27 |
| 0.50 |
-15.45 |
-989 |
-1399 |
4582 |
3.02 |
| 0.55 |
-13.92 |
-978 |
-1397 |
4743 |
2.90 |
| 0.60 |
-12.93 |
-971 |
-1413 |
4902 |
2.83 |
| 0.65 |
-12.22 |
-966 |
-1444 |
5058 |
2.77 |
| 0.70 |
-11.68 |
-961 |
-1486 |
5210 |
2.69 |
| 0.75 |
-11.23 |
-957 |
-1533 |
5357 |
2.53 |
| 0.80 |
-10.83 |
-953 |
-1576 |
5497 |
2.25 |
| 0.85 |
-10.46 |
-948 |
-1601 |
5630 |
1.82 |
| 0.90 |
-10.12 |
-943 |
-1592 |
5757 |
1.23 |
| 0.95 |
-9.79 |
-938 |
-1540 |
5879 |
0.58 |
| 1.00 |
-9.47 |
-934 |
-1455 |
6001 |
0.00 |
| PEG 200 + water |
| 0.00 |
-1306.70 |
-10455 |
-935 |
2456 |
0.00 |
| 0.05 |
-75.30 |
-1175 |
-1056 |
2783 |
-0.33 |
| 0.10 |
-71.54 |
-1108 |
-1122 |
3119 |
0.06 |
| 0.15 |
-66.10 |
-1081 |
-1190 |
3436 |
0.62 |
| 0.20 |
-59.54 |
-1068 |
-1270 |
3737 |
1.29 |
| 0.25 |
-52.47 |
-1062 |
-1370 |
4028 |
2.12 |
| 0.30 |
-45.37 |
-1060 |
-1500 |
4315 |
3.13 |
| 0.35 |
-38.60 |
-1062 |
-1668 |
4600 |
4.39 |
| 0.40 |
-32.36 |
-1065 |
-1882 |
4884 |
5.89 |
| 0.45 |
-26.77 |
-1066 |
-2119 |
5159 |
7.42 |
| 0.50 |
-21.86 |
-1057 |
-2297 |
5401 |
8.33 |
| 0.55 |
-17.63 |
-1034 |
-2294 |
5582 |
7.84 |
| 0.60 |
-14.02 |
-1003 |
-2092 |
5712 |
6.12 |
| 0.65 |
-10.96 |
-977 |
-1813 |
5831 |
4.17 |
| 0.70 |
-8.40 |
-959 |
-1565 |
5963 |
2.64 |
| 0.75 |
-6.27 |
-948 |
-1378 |
6112 |
1.59 |
| 0.80 |
-4.49 |
-942 |
-1243 |
6271 |
0.91 |
| 0.85 |
-3.03 |
-938 |
-1143 |
6435 |
0.48 |
| 0.90 |
-1.82 |
-936 |
-1063 |
6603 |
0.20 |
| 0.95 |
-0.83 |
-935 |
-995 |
6771 |
0.05 |
| 1.00 |
-0.02 |
-935 |
-936 |
6939 |
0.00 |
| PEG 400 + water |
| 0.00 |
-32.12 |
-1169 |
-935 |
2457 |
0.00 |
| 0.05 |
-139.34 |
-1709 |
-1719 |
3147 |
0.03 |
| 0.10 |
-107.70 |
-1422 |
-2013 |
3984 |
2.62 |
| 0.15 |
-77.87 |
-1232 |
-2026 |
4583 |
3.78 |
| 0.20 |
-55.12 |
-1117 |
-1929 |
5054 |
3.95 |
| 0.25 |
-39.03 |
-1049 |
-1806 |
5466 |
3.69 |
| 0.30 |
-27.92 |
-1008 |
-1691 |
5849 |
3.29 |
| 0.35 |
-20.24 |
-983 |
-1594 |
6216 |
2.87 |
| 0.40 |
-14.88 |
-968 |
-1514 |
6574 |
2.48 |
| 0.45 |
-11.10 |
-958 |
-1450 |
6925 |
2.14 |
| 0.50 |
-8.38 |
-952 |
-1399 |
7269 |
1.83 |
| 0.55 |
-6.39 |
-948 |
-1357 |
7607 |
1.56 |
| 0.60 |
-4.93 |
-945 |
-1322 |
7938 |
1.32 |
| 0.65 |
-3.83 |
-943 |
-1291 |
8262 |
1.10 |
| 0.70 |
-2.99 |
-941 |
-1262 |
8578 |
0.89 |
| 0.75 |
-2.35 |
-940 |
-1231 |
8887 |
0.69 |
| 0.80 |
-1.85 |
-939 |
-1197 |
9189 |
0.50 |
| 0.85 |
-1.46 |
-938 |
-1158 |
9484 |
0.33 |
| 0.90 |
-1.16 |
-936 |
-1116 |
9772 |
0.18 |
| 0.95 |
-0.91 |
-935 |
-1075 |
10054 |
0.07 |
| 1.00 |
-0.72 |
-935 |
-1038 |
10330 |
0.00 |
x1 is the mole fraction of polymeric cosolvent (1) in the {polymeric cosolvent (1) + water (2)} mixtures free of cyclosporin (3).
Figure 4 shows a non-linear variation of cyclosporin δx1,3 values regarding the polymeric cosolvent-proportion in the solvent mixtures as expressed by the mole fraction of every polymeric cosolvent before solute adding. Initially, the addition of DEGME to neat water as solvent makes negative the δx1,3 values of cyclosporin in the composition interval of 0.00 < x1 < 0.12. The maximum negative δx1,3 value is obtained in the mixture of x1 = 0.05 with δx1,3 = –1.28 × 10–2, being its absolute value slightly higher than 1.0 × 10–2. As indicated above, δx1,3 values over 1.0 × 10–2 are considered as consequence of real preferential solvation effects.49,50 Otherwise, in the case of aqueous-PEG 200 mixtures a negative δx1,3 value of –3.3 × 10–3 is observed which could be a consequence of uncertainties propagation toward IKBI calculations.49,50 Regarding preferential hydration in observed in the {DEGME (1) + water (2)} cosolvent system, it is probable that the structuring of water molecules by hydrogen-bonding around the methyl and methylene groups of this compound (Figure 1) conducting hydrophobic hydration contributes to lowering of the net δx1,3 to negative values in these water-rich mixtures.
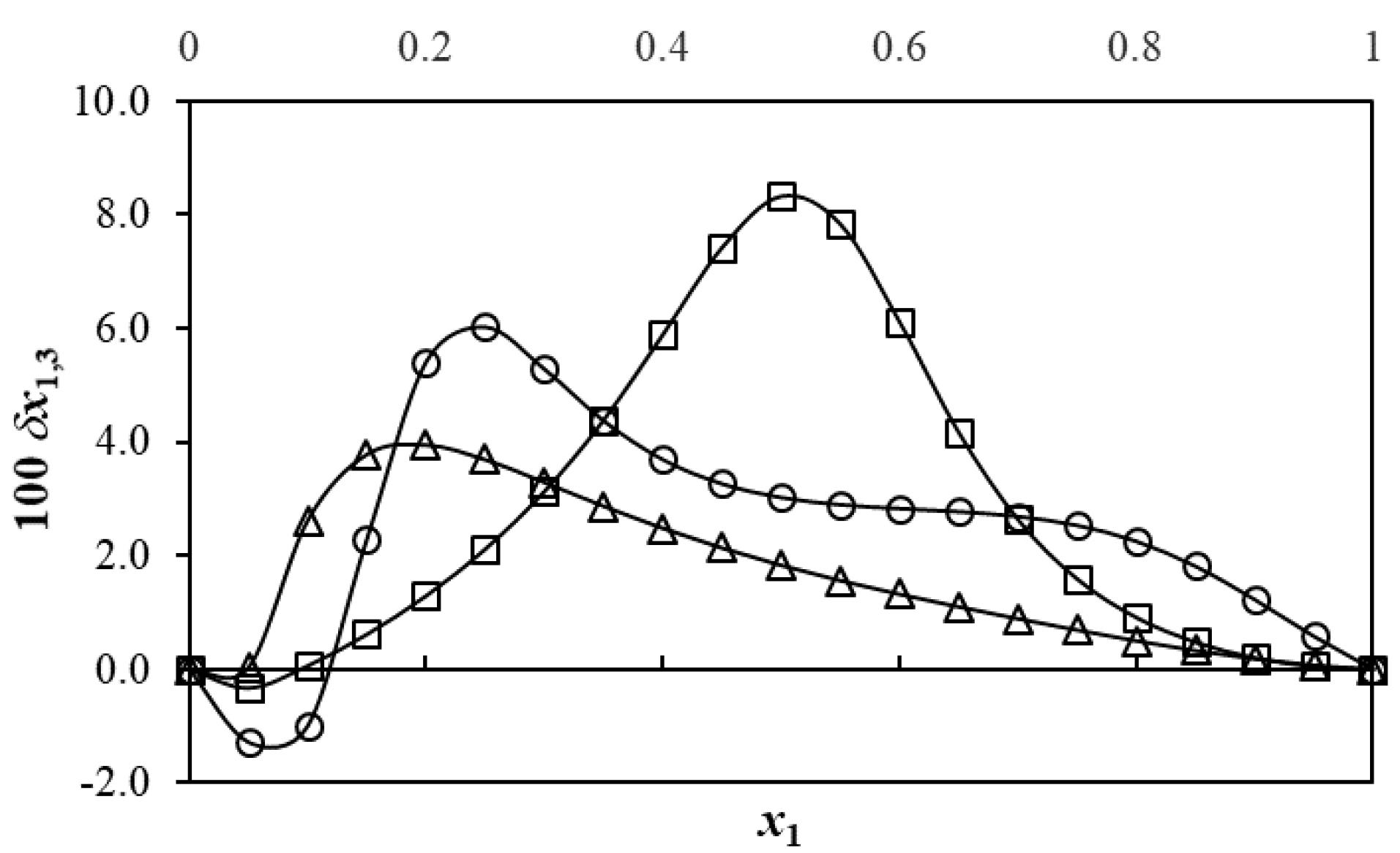
Figure 4.
Preferential solvation parameters (δx1,3) of cyclosporin (3) by polymeric cosolvent in some {polymeric cosolvent (1) + water (2)} mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); ∆: PEG 400 (1) + water (2)
.
Preferential solvation parameters (δx1,3) of cyclosporin (3) by polymeric cosolvent in some {polymeric cosolvent (1) + water (2)} mixtures at T= 298.15 K. ○: DEGME (1) + water (2); □: PEG 200 (1) + water (2); ∆: PEG 400 (1) + water (2)
In the mixtures composition interval of 0.12 < x1 < 1.00 the local mole fractions of DEGME around cyclosporin molecules are higher than those in the bulk aqueous- DEGME cosolvent mixtures in the absence of this drug. The maximum positive δx1,3 value is obtained in the mixture of x1 = 0.25, with δx1,3 = 6.02 × 10–2, which is higher than |1.0 × 10–2|. Hence, it could be considered as a consequence of preferential solvation effects of cyclosporin by DEGME molecules. For PEG 200 and PEG 400 aqueous mixtures δx1,3 values are positive in almost all the mixtures compositions reaching maximum positive δx1,3 values in the mixtures of x1 = 0.50 with δx1,3 = 8.33 × 10–2 for PEG 200-aqueous mixtures and x1 = 0.20 with δx1,3 = 3.95 × 10–2 for PEG 400-aqueous mixtures. In these mixtures composition intervals cyclosporin could be acting as a Lewis acid in front of the cosolvent polymeric molecules by means of its hydroxyl and primary amide groups (Figure 1), whose hydrogen atoms would be interacting with the unshared electron pairs of the oxygen atoms of polymeric cosolvent by hydrogen bonding. It is important to keep in mind that polymeric cosolvent exhibit a higher Lewis base behavior compared with water.42-44
Conclusions
Based on solubility values reported earlier,26 the preferential solvation parameters of cyclosporin in aqueous polymeric cosolvent mixtures were derived by means of the IKBI method at 298.15 K. Thus, this drug in DEGME-aqueous mixtures is preferentially solvated by water in water-rich mixtures but preferentially solvated by DEGME in mixtures of 0.12 < x1 < 1.00. In this way, it is possible that the preferential hydration in water-rich mixtures is due to hydrophobic hydration around methyl and methylene moieties (Figure 1). Otherwise, in PEG-aqueous mixtures cyclosporin is preferentially solvated by PEG in almost all the mixtures owing the more basic behavior of all these polymeric cosolvents compared with water. However, the specific cyclosporin-cosolvent or cyclosporin-water interactions are not well understood despite the thermodynamic treatment performed here owing the complexity of these ternary mixtures including the high molecular size of this drug.
Acknowledgements
We thank the Department of Pharmacy of the Universidad Nacional de Colombia for for facilitating computational resources.
Competing Interests
No potential conflict of interest was reported by the authors.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
Not applicable.
References
- Budavari S, O’Neil MJ, Smith A, Heckelman PE, Obenchain JR Jr, Gallipeau JA, et al. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th ed. Whitehouse Station, NJ: Merck; 2001.
- Sweetman SC. Martindale: The Complete Drug Reference. 36th ed. London: Pharmaceutical Press; 2009.
- Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol 2019; 163:472-80. doi: 10.1016/j.bcp.2019.03.022 [Crossref] [ Google Scholar]
- Survase SA, Kagliwal LD, Annapure US, Singhal RS. Cyclosporin A--a review on fermentative production, downstream processing and pharmacological applications. Biotechnol Adv 2011; 29(4):418-35. doi: 10.1016/j.biotechadv.2011.03.004 [Crossref] [ Google Scholar]
- Faulds D, Goa KL, Benfield P. Cyclosporin A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993; 45(6):953-1040. doi: 10.2165/00003495-199345060-00007 [Crossref] [ Google Scholar]
- Periman LM, Mah FS, Karpecki PM. A review of the mechanism of action of cyclosporine A: the role of cyclosporine A in dry eye disease and recent formulation developments. Clin Ophthalmol 2020; 14:4187-200. doi: 10.2147/opth.s279051 [Crossref] [ Google Scholar]
- Devaux CA, Melenotte C, Piercecchi-Marti MD, Delteil C, Raoult D. Cyclosporin A: a repurposable drug in the treatment of COVID-19?. Front Med (Lausanne) 2021; 8:663708. doi: 10.3389/fmed.2021.663708 [Crossref] [ Google Scholar]
- Hussain Y, Khan H. Immunosuppressive drugs. In: Rezaei N, ed. Encyclopedia of Infection and Immunity. Vol 4. Elsevier; 2022. p. 726-40. 10.1016/b978-0-12-818731-9.00068-9.
- Ismailos G, Reppas C, Macheras P. Enhancement of cyclosporin A solubility by d-alphatocopheryl-polyethylene-glycol-1000 succinate (TPGS). Eur J Pharm Sci 1994; 1(5):269-71. doi: 10.1016/0928-0987(94)90021-3 [Crossref] [ Google Scholar]
- Ran Y, Zhao L, Xu Q, Yalkowsky SH. Solubilization of cyclosporin A. AAPS PharmSciTech 2001; 2(1):E2. doi: 10.1208/pt020102 [Crossref] [ Google Scholar]
- Guo J, Wu T, Ping Q, Chen Y, Shen J, Jiang G. Solubilization and pharmacokinetic behaviors of sodium cholate/lecithin-mixed micelles containing cyclosporine A. Drug Deliv 2005; 12(1):35-9. doi: 10.1080/10717540590889691 [Crossref] [ Google Scholar]
- Malaekeh-Nikouei B, Nassirli H, Davies N. Enhancement of cyclosporine aqueous solubility using α- and hydroxypropyl β-cyclodextrin mixtures. J Incl Phenom Macrocycl Chem 2007; 59(3):245-50. doi: 10.1007/s10847-007-9321-4 [Crossref] [ Google Scholar]
- Czogalla A. Oral cyclosporine A--the current picture of its liposomal and other delivery systems. Cell Mol Biol Lett 2009; 14(1):139-52. doi: 10.2478/s11658-008-0041-6 [Crossref] [ Google Scholar]
- Zhao X, Zhou YQ, Potharaju S, Lou H, Sun HM, Brunson E. Development of a self-microemulsifying tablet of cyclosporine-A by the liquisolid compact technique. Int J Pharm Sci Res 2011; 2(9):2299-308. [ Google Scholar]
- Goyal R, Macri L, Kohn J. Formulation strategy for the delivery of cyclosporine A: comparison of two polymeric nanospheres. Sci Rep 2015; 5:13065. doi: 10.1038/srep13065 [Crossref] [ Google Scholar]
- Lahiani-Skiba M, Hallouard F, Bounoure F, Milon N, Karrout Y, Skiba M. Enhanced dissolution and oral bioavailability of cyclosporine A: microspheres based on αβ-cyclodextrins polymers. Pharmaceutics 2018; 10(4):285. doi: 10.3390/pharmaceutics10040285 [Crossref] [ Google Scholar]
- Mirzaeei S, Mohammadi G, Fattahi N, Mohammadi P, Fattahi A, Nikbakht MR. Formulation and physicochemical characterization of cyclosporine microfiber by electrospinning. Adv Pharm Bull 2019; 9(2):249-54. doi: 10.15171/apb.2019.028 [Crossref] [ Google Scholar]
- Wiącek AE, Jurak M, Ładniak A, Przykaza K, Szafran K. Cyclosporine CsA—the physicochemical characterization of liposomal and colloidal systems. Colloids and Interfaces 2020; 4(4):46. doi: 10.3390/colloids4040046 [Crossref] [ Google Scholar]
- Dubey P, Barker SA, Craig DQ. Design and characterization of cyclosporine A-loaded nanofibers for enhanced drug dissolution. ACS Omega 2020; 5(2):1003-13. doi: 10.1021/acsomega.9b02616 [Crossref] [ Google Scholar]
- Wu K, Gore A, Graham R, Meller R. Solubilization of cyclosporine in topical ophthalmic formulations: preformulation risk assessment on a new solid form. J Pharm Sci 2019; 108(10):3233-9. doi: 10.1016/j.xphs.2019.06.008 [Crossref] [ Google Scholar]
- Ghezzi M, Ferraboschi I, Delledonne A, Pescina S, Padula C, Santi P. Cyclosporine-loaded micelles for ocular delivery: investigating the penetration mechanisms. J Control Release 2022; 349:744-55. doi: 10.1016/j.jconrel.2022.07.019 [Crossref] [ Google Scholar]
- Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm 2017; 117:14-28. doi: 10.1016/j.ejpb.2017.03.006 [Crossref] [ Google Scholar]
- Ismailos G, Reppas C, Dressman JB, Macheras P. Unusual solubility behaviour of cyclosporin A in aqueous media. J Pharm Pharmacol 1991; 43(4):287-9. doi: 10.1111/j.2042-7158.1991.tb06688.x [Crossref] [ Google Scholar]
- Molpeceres J, Guzmán M, Bustamante P, del Rosario Aberturas M. Exothermic-endothermic heat of solution shift of cyclosporin A related to poloxamer 188 behavior in aqueous solutions. Int J Pharm 1996; 130(1):75-81. doi: 10.1016/0378-5173(95)04294-6 [Crossref] [ Google Scholar]
- Berton P, Mishra MK, Choudhary H, Myerson AS, Rogers RD. Solubility studies of cyclosporine using ionic liquids. ACS Omega 2019; 4(5):7938-43. doi: 10.1021/acsomega.9b00603 [Crossref] [ Google Scholar]
- Ha ES, Park H, Lee SK, Kang HT, Jeong JS, Kim MS. Equilibrium solubility, solvent effect, and equation correlations of cyclosporine in twenty mono solvents and four binary mixtures. J Mol Liq 2024; 399:124389. doi: 10.1016/j.molliq.2024.124389 [Crossref] [ Google Scholar]
- Rubino JT. Cosolvents and cosolvency. In: Swarbrick J, Boylan JC, eds. Encyclopedia of Pharmaceutical Technology. Vol 3. New York, NY: Marcel Dekker; 1988. p. 375-98.
- Martin AN, Bustamante P, Chun AH. Physical Chemical Principles in the Pharmaceutical Sciences. 4th ed. Philadelphia, PA: Lea & Febiger; 1993.
- Jouyban A. Handbook of Solubility Data for Pharmaceuticals. Boca Raton, FL: CRC Press; 2010.
- Allen LV Jr, Ansel AC. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. 10th ed. Philadelphia, PA: Wolters Kluwer; 2014.
- Rowe RC, Sheskey PJ, Quinn ME. Handbook of Pharmaceutical Excipients. 6th ed. London: Pharmaceutical Press; 2009.
- Hashemzadeh N, Jouyban A. Review of pharmaceutical applications of diethylene glycol monoethyl ether. J Pharm Pharm Sci 2022; 25:340-53. doi: 10.18433/jpps32921 [Crossref] [ Google Scholar]
- Marcus Y. Solvent Mixtures: Properties and Selective Solvation. New York, NY: Marcel Dekker; 2002.
- Marcus Y. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J Mol Liq 2008; 140(1-3):61-7. doi: 10.1016/j.molliq.2008.01.005 [Crossref] [ Google Scholar]
- Marcus Y. Preferential solvation of drugs in binary solvent mixtures. Pharm Anal Acta 2017; 8(1):1000537. doi: 10.4172/2153-2435.1000537 [Crossref] [ Google Scholar]
- Yalkowsky SH. Solubility and Solubilization in Aqueous Media. New York, NY: American Chemical Society, Oxford University Press; 1999.
- Marcus Y. Preferential solvation in mixed solvents. In: Smith PE, Matteoli E, O’Connell JP, eds. Fluctuation Theory of Solutions: Applications in Chemistry, Chemical Engineering, and Biophysics. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2013.
- Parra MA, Cerquera NE, Ortiz CP, Cárdenas-Torres RE, Delgado DR, Peña MÁ. Solubility of ciprofloxacin in different solvents at several temperatures: measurement, correlation, thermodynamics and Hansen solubility parameters. J Taiwan Inst Chem Eng 2023; 150:105028. doi: 10.1016/j.jtice.2023.105028 [Crossref] [ Google Scholar]
- Barton AF. Handbook of Solubility Parameters and Other Cohesion Parameters. 2nd ed. New York, NY: CRC Press; 1991.
- Ávila CM, Martínez F. Thermodynamic study of the solubility of benzocaine in some organic and aqueous solvents. J Solution Chem 2002; 31(12):975-85. doi: 10.1023/a:1021825509697 [Crossref] [ Google Scholar]
- Jiménez DM, Cárdenas ZJ, Delgado DR, Martínez F, Jouyban A. Preferential solvation of methocarbamol in aqueous binary co-solvent mixtures at 29815 K. Phys Chem Liquids 2014; 52(6):726-37. doi: 10.1080/00319104.2014.915755 [Crossref] [ Google Scholar]
- Martínez F, Jouyban A, Acree WE Jr. Comments on “Solubility and thermodynamic function of a new anticancer drug ibrutinib in {2-(2-ethoxyethoxy)ethanol + water} mixtures at different temperatures”. J Chem Thermodyn 2016; 95:180-2. doi: 10.1016/j.jct.2015.11.031 [Crossref] [ Google Scholar]
- Baracaldo-Santamaría D, Calderon-Ospina CA, Ortiz CP, Cardenas-Torres RE, Martinez F, Delgado DR. Thermodynamic analysis of the solubility of isoniazid in (PEG 200 + water) cosolvent mixtures from 27815 K to 31815 K. Int J Mol Sci 2022; 23(17):10190. doi: 10.3390/ijms231710190 [Crossref] [ Google Scholar]
- Mohammadian E, Rahimpour E, Martinez F, Jouyban A. Budesonide solubility in polyethylene glycol 400 + water at different temperatures: experimental measurement and mathematical modelling. J Mol Liq 2019; 274:418-25. doi: 10.1016/j.molliq.2018.10.088 [Crossref] [ Google Scholar]
- Fedors RF. A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci 1974; 14(2):147-54. doi: 10.1002/pen.760140611 [Crossref] [ Google Scholar]
- Douhéreta G, Salgado C, Davis MI, Loya J. Ultrasonic speeds and isentropic functions of 2-(2-alkoxyethoxy)ethanol + water at 29815 K. Thermochim Acta 1992; 207:313-28. doi: 10.1016/0040-6031(92)80145-m [Crossref] [ Google Scholar]
- Muñoz MM, Tinjacá DA, Jouyban A, Martínez F, Acree WE Jr. Volumetric properties of {PEG 200 (or 300) (1) + water (2)} mixtures at several temperatures and correlation with the Jouyban–Acree model. Phys Chem Liquids 2018; 56(1):100-9. doi: 10.1080/00319104.2017.1303700 [Crossref] [ Google Scholar]
- Rodríguez GA, Holguín AR, Martínez F, Khoubnasabjafari M, Jouyban A. Volumetric properties of (PEG 400 + water) and (PEG 400 + ethanol) mixtures at several temperatures and correlation with the Jouyban-Acree model. Rev Colomb Cienc Quim Farm 2012; 41(2):187-202. [ Google Scholar]
- Marcus Y. Solubility and solvation in mixed solvent systems. Pure Appl Chem 1990; 62(11):2069-76. doi: 10.1351/pac199062112069 [Crossref] [ Google Scholar]
- Ben-Naim A. Preferential solvation in two- and in three-component systems. Pure Appl Chem 1990; 62(1):25-34. doi: 10.1351/pac199062010025 [Crossref] [ Google Scholar]